The bulk physical properties such as strength and toughness of materials such as synthetic and natural polymers, or the activity and efficacy of proteins and biopharmaceuticals are strongly dependent on their molecular properties. In a large number of industries, there is a clear and strong desire to control molecular properties such as molecular weight and structure in order to better manipulate and control the bulk properties of the material. It is therefore necessary to have a reliable technique for making such measurements.
S ize E xclusion C hromatography (SEC), also referred to as G el P ermeation C hromatography (GPC) or G el F iltration (GF), is a separations technique and subset of H igh P erformance L iquid C hromatography (HPLC), whereby polymer molecules are separated based on their hydrodynamic volume. Sample molecules, dissolved in a suitable solvent, pass through a gel packing matrix within a column and diffuse into and out of pores within the gel. Smaller molecules diffuse more frequently and deeper into the pores of the packing matrix so that their progress through the column is impaired, whereas larger molecules penetrate fewer of the pores. In this way, molecules in a mixed sample, such as a polydisperse polymer or a mixture of protein molecules, are organized according to their size. After the separation, one or more detectors is used to characterize the sample. This always involves a concentration detector and may or may not include advanced detectors such as light scattering and intrinsic viscosity. The primary goal of this characterization is to measure the molecular weight and molecular weight distribution but may also include the measurement of other properties such as size and structure. The measured values can then be used to understand the sample’s bulk properties.
The bulk physical properties such as strength and toughness of synthetic and natural polymers, or the activity and efficacy of proteins and biopharmaceuticals are strongly dependent on their molecular properties. In a large number of industries, there is a clear and strong desire to control molecular weight and structure in order to better manipulate and control the bulk properties of the material.
For example, in polymer chemistry, different molecular parameters can have effects on different bulk properties. For proteins, molecular weight, and therefore, oligomeric state directly relates to their activity either as a biopharmaceutical, or in an assay. The amount and size of any aggregates present in a sample will result in a loss of sample activity and in the case of biopharmaceuticals, can also stimulate an immune response affecting the efficacy and safety of such drugs.
It is therefore necessary to have a reliable technique for making such measurements. Size Exclusion Chromatography (SEC), also referred to as Gel Permeation Chromatography (GPC) or Gel Filtration (GF) is defined as:
A separation technique in which separation, mainly according to the hydrodynamic volume of the molecules or particles, takes place in a porous non-adsorbing material with pores of approximately the same size as the effective dimensions in solution of the molecules to be separated.[i]
SEC is mainly used for the characterization of macromolecules including synthetic and natural polymers (including polysaccharides) as well as proteins or RNA/DNA. As SEC covers such a broad application range, the main focus areas are slightly different between applications. For synthetic and natural polymers the main purpose of the technique is typically for the determination of molecular mass averages and molecular mass distribution of the sample. For proteins, the main focus is typically the determination of monomeric and oligomeric states and their quantification. Further information, such as size, structural and compositional information, can be derived from multi-detector SEC-systems.
In addition to use at analytical level, SEC can also be applied at a preparative scale for large-scale separations or purification purposes (whereby eluting molecular weight fractions can be collected in a suitable container and isolated). SEC can also be applied to solid matter (nanoparticles) dispersed in a liquid. Despite the increasing interest in nanoparticle characterization, SEC studies on nanoparticles still form only a minor area of interest and the main focus of SEC remains on characterization on a molecular level.
SEC is a liquid chromatography (LC) method, a subset of HPLC, and requires the sample material to be completely dissolved with the individual molecules dispersed and not interacting. In certain cases, where the aim is to study assemblies of molecules, e.g. complexes of several proteins, conditions have to be chosen to keep those complexes intact. Although dissolution is straightforward for a lot of samples, care has to be taken to make sure samples do go into solution completely and do not degrade or get modified by the dissolution process.
The columns used for SEC are filled with a gel matrix containing highly porous spherical particles. The most common materials used in these gels include cross-linked polymers like polystyrene, acrylates, dextran or silica. A constant eluent flow is forced through the column by an isocratic pump. The sample solution is introduced into the flow by means of an injection valve and loop.
Under ideal conditions, there is no interaction between the sample and the gel, meaning that the separation process should be based purely on diffusion of the analyte while the solution travels through the stationary phase.
If there is no adsorption of the analyte to the stationary phase, the separation process is purely entropically driven. The standard free energy change ∆G° of a chromatographic process is generally described by
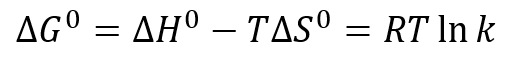
with ∆H° being the change in standard enthalpy, ∆S° the change in standard entropy, R the gas constant, T the absolute temperature and k the partition coefficient. With the separation being free of enthalpic contributions, the above simplifies for SEC to
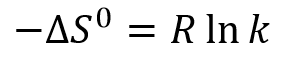
This shows that the separation process is not expected to be temperature-dependent. In practice, temperature can be seen to have a small effect on the result due to its effect on solvent viscosity and the molecules’ diffusion rates.
Furthermore, the flow rate dependency of the separation process is also limited: the flow has to be faster than the re-mixing of components due to backwards diffusion of previously separated molecules, but it should not be too fast to allow time for the separation process to happen. Also, the column packaging material sets an upper limit to the usable flow rates. Some samples might degrade during the passage through the column if too high flow rates are applied due to the shear forces within the liquid. Typically, flow rates for analytical SEC systems are in the range of 0.1 to 1.0 mL/min.
To achieve a purely diffusion driven separation process, a suitable combination of mobile and stationary phase is required where there is no interaction between the two. The analyte molecules will then diffuse in and out of the pores of the packaging material as the mobile phase carries the sample through the column. Whilst a molecule is inside a pore of the packaging material, its passage through the column will be delayed compared to the other molecules. Since smaller molecules will find their way into more of the pores in the packing gel, and also penetrate more deeply, they will be delayed more than larger molecules. Thus the result is a separation of the molecules according to their hydrodynamic size with the larger molecules eluting first.
Figure 1 shows the separation process (schematic) from the time where the sample is introduced into the column until the sample eluted completely.
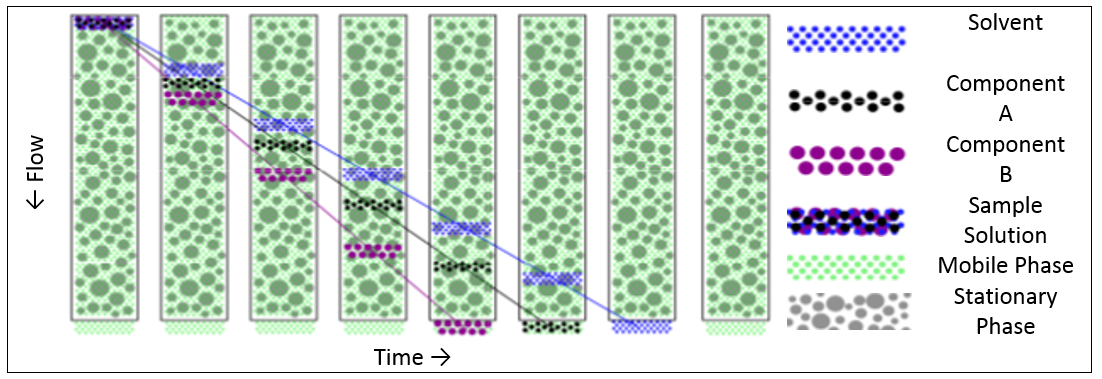
Figure 1: SEC separation process (schematic)
Clearly there are limits to the separation at the extreme ends of the size separation. Any molecules that are larger than the largest pores will not experience any delay in elution and therefore will not be separated from each other. These will elute together having travelled only through the interstitial volume of mobile phase between the packaging particles of the column (around 14 mL in the example shown in Figure 2). This is called the “void volume”.
Any molecules which are smaller than the smallest pores will diffuse into all of the pores in the gel packing matrix and will also not be separated from each other. These will be the last molecules to elute (around 34 mL in the example shown in Figure 2). Since almost every injection will include solvent or salt molecules which are in this size range, a peak in this region will always be visible on the RI detector and is often called the ‘solvent peak’. The elution of this peak marks the end of the measurement (called “total permeation volume”, VT) and it is worth noting that this is therefore independent of the sample. It can be used to determine the sum of interstitial volume and intra-particle volumes. The intermediate volume where analyte molecules are able to partially penetrate the column packaging represents the useful range for SEC separation. Many columns and pore sizes are available to tailor the separation range to the specific application.
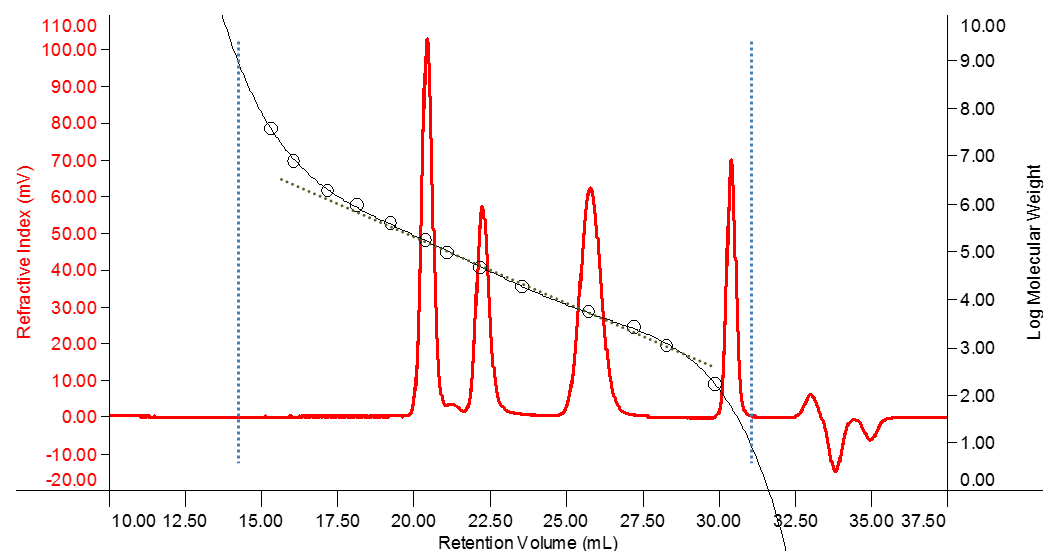
Figure 2: Conventional Calibration Curve
Figure 2 shows the refractive index detector trace (red) of a mixture of three polymer standards eluting at about 20.5, 22.5 and 26 mL retention volume. By running a series of standards with known molecular mass, a calibration curve can be determined which links the standard material’s mass to its retention volume. The standards measured in this example are represented by the circles and the sigmoidal curve (black) fitted through those points represents the calibration curve.
The linear fit through the calibration curve in Figure 2 shows that there is a region where log(M) is almost linear with respect to retention volume. The slope of the calibration curve gradually increases towards the higher and lower masses at the end of this linear region. This is because the size of the analyte molecules is approaching the upper and lower limits of the gel pores and the separation starts to break down. If a significant fraction of the sample elutes in these regions, the column choice may not be ideal for the sample under investigation. A column with smaller or larger pores might be required to optimize the separation.
Column dimensions in analytical SEC applications are typically 8 mm inner diameter and 30 cm length. Some larger variants are available for (semi-) preparative work and smaller variants are available which are predominantly used in areas with precious samples or expensive solvents.
The column beds are made from various materials and their choice depends on the sample/solvent combination. Standard materials include cross-linked polystyrene (styrene-co-divinyl benzene, SDV), acrylic polymers, polyvinyl alcohol and silica. Other packaging materials (such as cross-linked sugars) are available for more specialized requirements. The columns used must be compatible with the solvent used for the sample and must not exhibit significant interaction with the analyte molecules.
One or more columns may be used for a separation with a greater number of columns increasing the resolution of the measurement. Each column adds additional pores, improving the separation; however this is subject to diminishing returns as each additional column provides less additional separation. One or two columns in series is the most common setup, however up to six or more may be used in some circumstances.
Two parameters can be easily used to describe the behavior of a column: the theoretical plate number and the slope of the linear part of the conventional calibration curve.
The theoretical plate number is measured by injecting a small molecule sample into the system. The width and retention volume of the resulting peak is influenced by changes in the volumes of the column, changes to the column bed such as fractures or the formation of voids, and could even be influenced if there is a change in the way the column surface interacts with the molecule used for testing. So one could say that the theoretical plate number monitors the “health” of the whole column bed. For calculation of the theoretical plate number see Malvern Panalytical's Technical note “Determination of Theoretical Plate Numbers in SEC/GPC” for details.[ii]
The slope of the linear part of the calibration curve (about 17 to 28 mL retention volume for the example shown in Figure 2) defines how well macromolecules of different sizes are separated. If standards are measured for a conventional calibration, the easiest way to asses this is to create a first order fit calibration including only the relevant standards. The software will then determine the calibration function in a format like log10 M = a · Vretention + b (where a represents the slope of the calibration line). Another way of monitoring this parameter for a given column set is to check the retention volumes for a large and small macromolecular sample. If those change, it is time to check the health of the column more thoroughly.
A basic SEC system consists of an isocratic pump optimized to deliver a constant flow over time, an injection valve, SEC-column(s) and a concentration detector. Most systems are also equipped with an online-degasser and autosampler, the latter for more convenient system operation. Degassing the solvent prevents spontaneous degassing and formation of bubbles which would lead to unstable flow and false detector signals. A column oven will help to maintain stable conditions for the separation process to ensure reproducibility.
The most important property of a concentration detector used for SEC is that the response is independent of the molecular mass of the analyte.
The RI detector is by far the most common and versatile concentration detector for SEC. The response to the change in the refractive index of the solution versus the pure solvent is linear at low concentrations. Most sample-solvent combinations show a change in refractive index of the solution with concentration. If no signal can be detected, the sample-solvent system is called isorefractive. The only way to get round this problem will be using a different solvent.

Figure 3: Differential Refractive Index Detector - working principle
The schematic in Figure 3 shows the basic components for an RI detector. A light source on the left shines light onto a flow cell. The flow cell contains two compartments shown in blue and green, one for the pure mobile phase (typically stationary in normal operation) and the other for the eluent flowing through the detector. If there is a refractive index difference between the compartments, the light will not go straight through the cell as the interface between the two cell compartments is tilted towards the optical axis. This leads to a deflection of the light which is then detected. The deflection is proportional to the analyte concentration in the flow cell and a factor, the so-called refractive index increment dn/dc, which describes the dependency of the solution’s refractive index from the concentration.
The dn/dc depends on the chemical composition of the sample and the eluent. In most cases the dn/dc is positive but there are also some cases with negative values (e.g. silicone in toluene).
The refractive index of the eluent also depends upon temperature and pressure. Therefore it is important to maintain a very stable temperature within the detector. The pressure within the cell is dependent on the pump, which is usually stable enough in the case of modern dual piston pumps.
The other common concentration detector for SEC is a UV detector. If analyte molecules absorb light within the UV light wavelength range, this absorbance can be measured. In accordance with the Beer–Lambert–Bouguer law the concentration of the analyte can be determined from the absorption if the attenuation coefficient of the analyte is known. In SEC applications, this is usually referred to as the dA/dc value, however in protein research, the same value is known as ε0.1% value. See Malvern Panalytical’s technical note “What is dA/dc?” for further information.[iii]
UV absorption at multiple wavelengths or in combination with RI detection allows in some cases determination of compositional information. For example, UV detection at 254 nm in combination with RI detection for compositional analysis of copolymers like poly butadiene-co-styrene, or UV detection at 280 and 260 nm for checking on purity or composition of protein/DNA samples. Such questions promoted the use of diode array UV detectors in SEC.
The main objective for SEC/GPC analyses is to find out about molecular mass and mass distribution of the analyte. Polymers are polydisperse systems, containing molecules of different chain lengths within one sample. Proteins are typically monodisperse (every molecule has the same sequence of amino acids and hence the same mass) but they can form oligomers and aggregates in solution. For these reasons, molecular mass measurements by SEC/GPC are given as average values. There are different moments defined:
Mn is the number-average molecular mass:
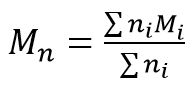
Mw is the mass-average molecular mass:
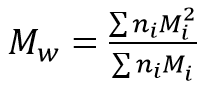
Mz is the z-average molecular mass:
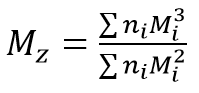
with Mi being the mass of the molecules, I, and ni being the number of molecules being measured. The ratio Mw / Mn is referred to as polydispersity of the sample, and is a measure of the width of the molecular mass distribution.
There are different ways to determine the Mi and ni values.
Conventional calibration is the easiest and most widely applied method to determine the molecular mass averages from a SEC/GPC analysis. The molecular weight separation range of the column is calibrated using standards of known molecular mass, resulting in a calibration curve as shown in Figure 2. The analyte’s concentration (ni) at every point, or data-slice, on the chromatogram is measured using the detector response. The molecular weight at each point (Mi) is calculated from the calibration curve and the molecular weight distribution is calculated using the equations above.
The downside of this simple approach is that the reported molecular mass is only relative to the standards used to generate the calibration curve. The result is valid only if the structure and composition of analyte and standard are the same, e.g. unbranched polystyrene. Differences in the molecular structure (branching or changes in conformation) or composition of the analyte result in deviations between actual and reported mass.
In universal calibration, the SEC/GPC system not only requires a concentration detector but also a viscometer detector. Again, the molecular weight separation range of the column is calibrated with standards, but this time, the product of intrinsic viscosity [η] and molar mass M. Log [η]·M is plotted as a function of the sample’s retention volume. The intrinsic viscosity is related to the molecule’s density in solution. It has been shown that calibration curves overlay for many different types of polymers by this approach. Therefore, it can be used to determine the true molecular mass of the sample in many cases.
The sample’s molecular weight is then calculated from its measured concentration and intrinsic viscosity and the molecular weight distribution is then calculated in the same way as above.
In a process called Static or Rayleigh light scattering, a molecule can absorb and re-emit a photon without loss of energy on the photon. The intensity of the scattered light is proportional to the molecular mass of the molecules under investigation. This proportionality allow calculation of the absolute molecular mass of the analyte molecules.
For more information on measurements of molecular weight using light scattering, refer to Malvern Panalytical’s white paper “Static Light Scattering Technologies for GPC/SEC explained. iv
It is also possible to determine some information about the sample composition from a SEC/GPC experiment.
If two concentration detectors are used, such as RI and UV, and components of the analyte exhibit different responses to these detectors, it is possible to determine the percentage of co-monomers in a co-polymer or the composition of conjugated (e.g. PEGylated) proteins.
If the sample’s intrinsic viscosity has been measured with a viscometer detector and the true molecular mass has been computed, then by means of the Mark-Howink-Plot (log [η] vs log M) and application of the Zimm-Stockmeyer theory it is possible to compute information about the branching in the sample.
SEC/GPC is an invaluable tool for the measurement, primarily, of molecular weight and molecular weight distribution. By separating molecules of a synthetic or natural polymer, or protein, it is possible to characterize them to high levels to generate important information about the molecules in the samples, which can then be related back to its bulk physical properties. Advanced detectors can increase the value of this technique by generating more accurate information such as absolute molecular weight or providing additional information, on structure, branching and composition.
[i] http://goldbook.iupac.org/S05705.html as of 5 June 2015
[ii] Determination of Theoretical Plate Numbers in SEC/GPC - Technical Note
[iii] What is dA/dc? - Technical Note
[iv] Static Light Scattering - page