The Zetasizer Nano ZS is used to compare three laundry detergents. Size measurements show a multimodal distribution indicating the presence of micelles and additives. Their zeta potentials, though very different are all highly negative.
Three commercial laundry agents produce size distributions with a main micelle peak near 10nm, a larger peak near 200nm (probably carboxyl methyl cellulose), and two of these consumer products showed an additional peak in the micron region. This is speculated to be very large molecular weight additives to reduce redeposition of dirt back onto the clothes. The average zeta potential of the detergent with the smallest micelles was -55mV, the one with the largest micelles showed -38mV, indicating strong formulation stability in all cases.
Product performance, product ageing, consistency and robustness of the final product properties are key objectives during the development of consumer products. Product performance issues are continuously changing because they include changing consumer expectations, regulatory requirements and developing environmental concerns.
The removal of dirt from clothing during washing is facilitated by surfactants. Surfactants are molecules with a hydrophilic, or polar group and a hydrophobic, or non-polar, chain on the same molecule. The hydrophilic side of the molecule "likes" water, while the hydrophobic side of the molecule avoids water.
This molecular structure means that surfactants can exist as isolated molecules at low concentration but also self assemble into various shapes at higher concentrations. These assemblies can form monolayers or self assemble into spherical structures, called micelles (Figure 1). The self assembled structures occur at a specific concentration known as the critical micelle concentration or cmc.
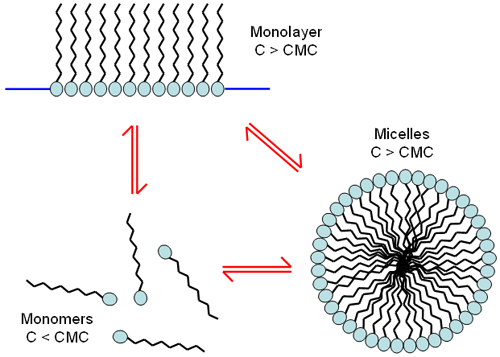
|
An example of this is a water droplet coated with surfactant. The hydrophobic side of the surfactant molecule will migrate to the air interface and the hydrophobic side will assemble into an ordered layer and stick out of the water surface. When this happens the hydrophobic side of the surfactant molecule is preferentially located on the surface, at the liquid-air interface. Without water molecules at the surface the surface tension decreases. Therefore, the water no longer beads up, but spreads and foam formation is also possible. The hydrophobic side of the molecule has an affinity for any grease, fat, or oil on the surface, aiding the spread of the liquid drop.
Micelles themselves are not directly related to detergency (dirt removal). The surfactant's role is to solubilize oily material and that ability is based on the unassociated surfactant molecule's ability to adsorb to the dirt (solid) to water interface. Consequently, adsorption at the air to water interface will lower the surface tension and cause foaming, but foam creation is not related to detergent effectiveness. In fact, detergency is unaffected by the presence of micelles, other than as reservoirs for replenishing the unassociated surfactant adsorbed from solution. Although most good detergents are made up of surfactants that aid good micelle formation; the actual formation of a micelle is a competing mechanism to the cleaning action.
Soap is made from a fatty acid that is reacted with an alkali. The acid end of the fatty acid reacts with the alkali to form a salt that is water-soluble. The other end is the fatty end, which repels water, and is attracted to fats and oils, i.e. it is non-polar or hydrophobic tail of the molecule. The process of making soap is called saponification. One of the earliest soaps used by mankind was sodium stearate, which was derived from beef fat that was reacted with alkaline wood ashes. This was the cleaning product of choice throughout history.
German scientists created the first detergents during World War II. These molecules were branched-chain alkyl benzene sulfonates. Like the earlier sodium stearate soap, these synthetic alkyl benzene sulfonates could precipitate hard minerals out of water. The removed minerals formed a scum.
Although these alkyl benzene sulfonates were used for many years, they created environmental problems. Microbes in the environment could not break down the branched-chain types of detergents and that left foam scum in rivers. Therefore, the next step in the evolution of detergents was to replace the branched-chain detergents with straight-chain alkyl benzene sulfonates. Examples of these molecules include sodium dodecylbenzinesulfonate and sodium xylenesulfonate.
However, these straight-chain detergents also had issues, i.e. they did not work in hard water. To remove these problems, phosphates were added to detergents to soften the water, but phosphates are excellent fertilizer for algae in rivers and oceans. These algae blooms began to deplete the oxygen in the water, killing fish so phosphates were replaced with other water softeners such as sodium carbonate and EDTA (Ethylene Diamine Tetra Acetic acid, a chelating agent).
Much more recently, surface-acting polyglucosides have been promoted as cleaning agents. These sugar-based detergents are easily broken down by microbes, leaving no traces in the environment. They consist of a pair of glucose molecules, with hydrocarbon side chains attached to act as the hydrophobic ends. They are milder than soaps, and work in hard water.
Another type of detergent is a group called the pyrrolidones. These are complex molecules that dissolve in both water and organic solvents.
Some laundry detergents contain fluorescent dyes that glow blue-white in ultraviolet light; they are used as "optical brighteners." The blue-white color from these dyes makes a yellowed fabric appear white.
Laundry detergent also might contain polyethylene glycol which is a water soluble polymer that prevents dirt from re-depositing on the clothes. Another polymer used for this purpose is carboxy methyl cellulose which is derived from natural cellulose and is also very water soluble.
Additional laundry detergent ingredients include Diethyl Ester Dimethyl Ammonium Chloride (DEEDMAC). This is used in the formulation as a fabric softener, but primarily it is a cationic surfactant that is extremely biodegradable. It works by reducing the friction between fibers, as well as between the fabric and the skin. Cationic surfactants are molecules where the hydrophilic part is positively charged. These molecules often contain an ammonium group attached to a halogen, as in this ammonium chloride example. Because they are cationic they are attracted to surfaces that are negatively charged, such as proteins and many synthetic fabrics.
Anionic surfactants, such as those making up the basic soap composition, often have a sodium, potassium, or ammonium group counterion, as in the original sodium stearate detergent.
Non-ionic surfactants like polyethylene glycol esters (PEG) are used as mild cleansers, or to add viscosity, to thicken the product mixture.
Amphoteric surfactants are those that have an acid and a base in the same molecule and are primarily used in cleaning products to stabilize foam and thicken the mixture. At a specific pH, amphoteric surfactants can also be referred to as zwitterionic. A zwitterionic surfactant means that the surfactant has an overall neutral charge.
Examples of detergent type surfactants are:
1.) Ammonium Lauryl Sulfate
2.) Lauryl Glucoside
A series of commercially available laundry detergents were purchased and dispersed in tap water at a concentration of 0.3 wt%. The tap water in this specific case had a pH of 6.5.
Size measurements were obtained by dynamic light scattering (DLS). These hydrodynamic diameter (Dh) measurements were carried out using a Zetasizer Nano ZS instrument from Malvern Instruments.
Zeta potential measurements were carried out in standard disposable capillary cuvettes (DTS1060) also using a Zetasizer Nano ZS instrument from Malvern Instruments. The disposable capillary cuvettes are reusable; in the case of these studies two cuvettes were reused after simple rinsing with deionized water between each measurement.
Table 1 indicates the size and zeta potential results obtained on three popular commercial liquid laundry detergents. Laundry Detergent A results in a bimodal distribution while Laundry detergents B and C each result in trimodal distributions. It is believed that samples labelled Laundry Detergent B and C are using two different additives, large molecular weight molecules, to reduce the redeposition of dirt back onto the clothes during the wash cycle.
| SAMPLE | Mean Diameter, Peak (1)
[nm] | Mean Diameter, Peak (2)
[nm] | Mean Diameter, Peak (3)
[nm] | Avg Zeta Potential
[mV] |
|---|---|---|---|---|
| Laundry Detergent A | 10.4 | 121.3 | --- | -38 |
| Laundry Detergent B | 7.6 | 188.7 | 5175 | -46 |
| Laundry Detergent C | 6.6 | 344.4 | 5193 | -55 |
Zeta potential results in Table 1 also indicate that all three of these commercial detergents have a high negative zeta potential. Laundry Detergent A, B and C each result in average zeta potential values of -38, -46, -55mV respectively. These are in the range of zeta potential values that would be expected for various anionic laurel sulfate surfactants that might be used in these products.
Figure 2 shows an example particle size distribution result of Laundry Detergent A. This commercial laundry detergent is found to have a bimodal distribution. The small particle size distribution (approximately 10nm) is associated with the surfactant-micelle size while the larger size distribution is associated with the detergent additives (approximately 120nm), which in this specific case is probably Carboxy Methyl Cellulose.
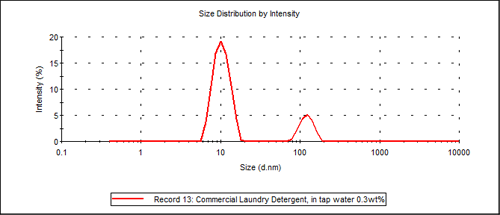
|
Figure 3 shows an overlay of the two trimodal particle size distributions obtained for Laundry Detergent B and Laundry Detergent C.
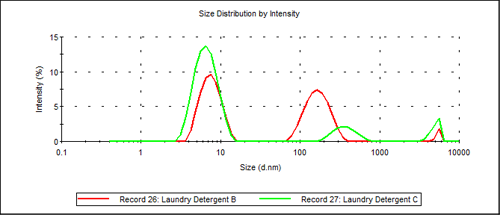
|
The key difference between the two results is that the primary additive in Laundry Detergent C has a diameter of 344nm almost twice the diameter of the additive used Laundry Detergent B.
Overlaid zeta potential results are shown in Figure 4 for all three laundry detergent samples (A, B and C). All three samples are based on highly negatively charged anionic surfactant.
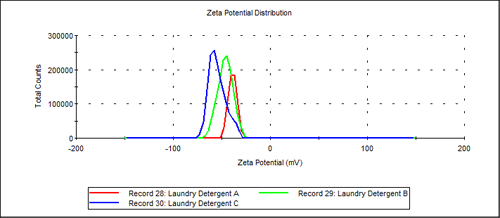
|
Consumer products are made up of multicomponent formulations that are continuously evolving as environmental problems arise and consumer preferences change. From an initial soap composition of sodium stearate, derived from beef fat reacted with alkaline wood ashes, these consumer goods have evolved to linear chained anionic surfactant such as alkyl benzene sulfonates and more natural compositions of alkyl glucosides made from oils and sugars.
Product performance, product ageing, consistency and robustness of the final product properties are key objectives during the development of consumer products. Product performance issues are continuously changing because they include changing consumer expectations, regulatory requirements and developing environmental concerns.
In detergents, micelle size and charge are key properties that may be used to assess product performance such as solubility at high/low temperatures and performance at the different pH's of water supplies. The Zetasizer Nano is an easy-to-use tool to provide insight into surfactant behavior at various solution conditions to help determine consistent detergent performance.
No matter what the formulation, consumers continue to require that clothes be clean, dishes be spotless, cars be bright and their hair be shiny. Soap was probably discovered just after cooking on an open fire began, i.e. as food fats splashed onto the ashes of the fire.
Current day soap products have evolved to require not only the properties of cleanliness but that our clothes or other surfaces smell fresh, never look yellow and also that the soap be biodegradable and environmentally friendly in all stages of use.
1.) Duncan J. Shaw, Introduction to Colloid and Surface Chemistry, 3rd Ed. Butterworths (1980) p 138-143.
2.) Milton J. Rosen, Surfactants and Interfacial Phenomena, Wiley (1978).