The Zetasizer Nano is used to measure the size of a polyelectrolyte-surfactant complex at a range of pH values, which indicates a reversible change in complex structure at low pH. A series of novel polyelectrolyte-surfactant complexes were studied in order to probe several candidates as potential pH sensitive materials. At neutral pH, the complexes are water soluble, rod-like nanorods. However, gelation and longer-range interactions are observed as the pH is dropped to low values (approximately pH 1). This is proposed to be a change in the complex structure, and dynamic light scattering (DLS) is used to determine changes in the overall complex size in dilute solutions. Currently, since the shape is not known, the translational diffusion coefficient that is measured in DLS is used to calculate an effective hydrodynamic diameter of the complex. The hydrodynamic diameter is measured as the pH of the solution is reduced from neutral to acidic condition, ranging between 180nm down to 50nm hydordynamic diameter.
Viet Lam, Lynn Walker, Dept. of Chem Eng, Carnegie Mellon University, Pittsburgh
Polyelectrolyte-surfactant complexes are used in numerous applications ranging from detergents (for solubilization of oils) to more exotic applications like the molecular design of templates for nanomaterials. These self-assembled aggregates are extremely versatile, robust and often inexpensive. However, understanding the effect of the tenuous interactions that define the complexes (electrostatic-hydrophobic balance) is vital to the design and success of these materials in current and future applications.
A series of novel polyelectrolyte-surfactant complexes were studied in order to probe several candidates as potential pH sensitive materials. At neutral pH, the complexes are water soluble, rod-like nanorods. However, gelation and longer-range interactions are observed as the pH is dropped to low values (approximately pH 1). This is proposed to be a change in the complex structure, and dynamic light scattering (DLS) is used to determine changes in the overall complex size in dilute solutions. Currently, since the shape is not known, the translational diffusion coefficient that is measured in DLS is used to calculate an effective hydrodynamic diameter of the complex. The hydrodynamic diameter is measured as the pH of the solution is reduced from neutral to acidic conditions.
DLS measurements were made on a Malvern Zetasizer Nano S with a detection angle of 173° using a 4mW He-Ne laser operating at a wavelength of 633nm. The polyelectrolyte-surfactant complexes were synthesized at Carnegie Mellon University and freeze dried for storage. Samples are later reconstituted in filtered deionized water to prepare for measurement. The polyelectrolyte has a molecular weight of approximately 350K g/mol and the resulting polyelectrolyte-surfactant complex has a molecular weight on the order of 1,000,000 g/mol.
Figure 1, shows the hydrodynamic diameter measurement results for a series of polyelectrolyte-surfactant complexes in solution at different values of pH. In this case, low concentrations were used in order to extract hydrodynamic data on individual particles without any concerns about interparticle interactions. A sharp increase in overall complex size is observed as the pH drops below 2, consistent with the observation of gelation at higher concentrations. This phenomenon is reversible and is not due to inter-complex interactions. This explanation was excluded by diluting the complexes at various values of pH and finding no concentration dependence on size, which verifies that the change is actually a change in the complex size. This additional information suggests a change in structure which will be verified with other techniques (cryo-TEM or SANS).
|
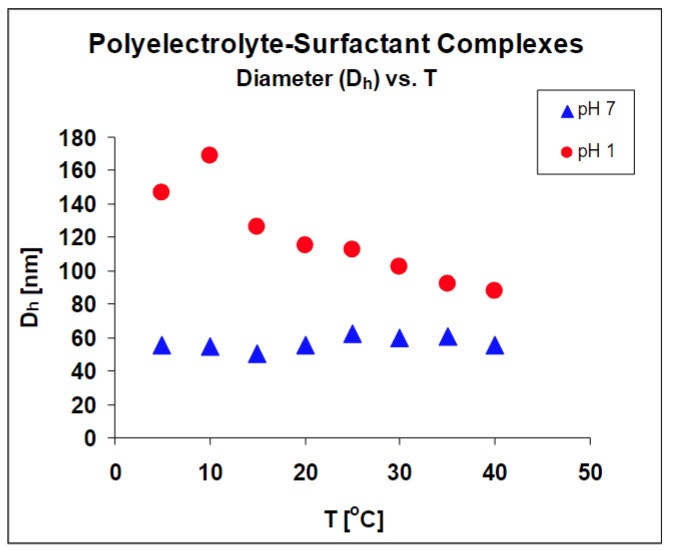
The impact of temperature on the complex structure provides information about the relative strengths of electrostatic and hydrophobic interactions. Results shown in figure 2, indicate that the complex structure is not a function of temperature at neutral pH, but is a strong function of temperature at low pH. The structural changes in complex size as stated before are reversible and reproducible. These results are being used to develop a structural model for these materials.
|
The DLS results verify that the balance of the interactions controlling complex structure, change at low pH, indicated by the change in temperature dependence.
1. Loxley, A. and B. Vincent, Equilibrium and kinetic aspects of the pH-dependent swelling of poly(2-vinylpyridine-co-styrene) microgels. Colloid & Polymer Science, 1997. 275(12): p. 1108-1114.
2. Yin, J., et al., pH-Induced Deswelling Kinetics of Sterically Stabilized Poly(2-vinylpyridine) Microgels Probed by Stopped-Flow Light Scattering. Langmuir, 2008.
3. Gerber, M.J., S.R. Kline, and L.M. Walker, Characterization of rodlike aggregates generated from a cationic surfactant and a polymerizable counterion. Langmuir, 2004. 20(20): p. 8510-8516.
4. Kuntz, D.M. and L.M. Walker, Solution behavior of rod-like polyelectrolyte-surfactant aggregates polymerized from wormlike micelles. Journal of Physical Chemistry B, 2007. 111(23): p. 6417-6424.
5. Lam, V.D., Polyelectrolyte-surfactant complex - a pH induced transformation from rod-like to string-of-pearl structures, in preparation.