Dr Steve Tonge, Andrew Harper, Malvern Cosmeceutics Ltd., Malvern Hills Science Park
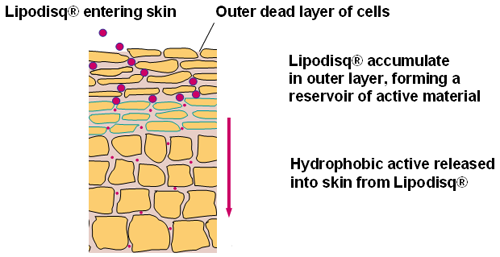
Lipodisq® (Malvern Cosmeceutics Ltd.) are novel lipid/polymer or surfactant nanoparticles that have been developed as mimics of naturally-occurring high density lipoproteins (HDL). They have been designed for use in topical delivery systems to transport hydrophobic (oil -soluble) pharmaceutical and cosmetic 'active' agents.
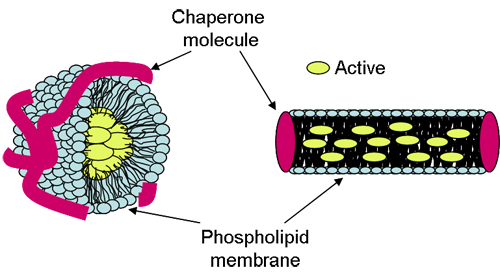
Lipodisq® particles are in the size range of 10 to 40nm diameter enabling enhanced penetration and diffusion into the skin resulting in improved topical drug delivery [1,2]. The particles are composed of a phospholipid membrane core conformed by a responsive chaperone molecule and are believed to exist in either spherical or discoidal form (Figure 1).
|
|
Lipodisq® can be readily formed with chaperone molecules of differing structure which spontaneously associate with phospholipids to create macromolecular assemblies. The size and shape of the chaperone molecule is a critical factor in Lipodisq® formation and also imparts the properties of the particle, i.e. particle size and/or its biodegradability. A unique feature of the Lipodisq® system is the ability to precisely control particle size at the nanometer level ~1nm by a structural modification of the chaperone molecule through alteration of pH in the case of the polymer-based system or temperature in the case of the surfactant-based system.
The pH at which Lipodisq® formation occurs can be carefully modified by varying the ratio of hydrophobic to hydrophilic groups along the polymer backbone of the chaperone molecule.
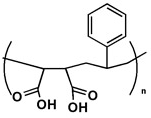
Poly(styrene-maleic acid) (Figure 2) is a suitable chaperone molecule. In its charged form, the copolymer chain is extended by charge repulsion; as the pH is lowered, charge is lost and the structure collapses into a compact coil. At a critical pH point during the coiling phase, the macromolecular Lipodisq® assemblies described above will begin to form spontaneously.
|
The pH at which copolymer/phospholipid association occurs, known as the critical collapsing pH, is dependent upon the attainment of a particular Hydrophilic/Lipophilic Balance (HLB) within the copolymer chains. It is therefore possible to tailor the ratio of the styrene and maleic acid monomer units such that the polymer interacts with lipid over a defined pH range, thereby enabling the selection of a styrene-maleic acid copolymer which is ideally suited for a specific e.g. physiological, pH range.
Subtle adjustments of the pH to a value below the critical collapsing pH will result in the relaxation of the chaperone molecule and a concomitant increase in the particle size; a mechanism which enables particle size to be precisely controlled.
Poly(styrene-maleic acid) Lipodisq® solutions can be manufactured which are stable at pH 4, 6, 7 and 9. Such solutions can be used in conventional pharmaceutical and cosmetic gels and lotions, and have also been employed to solubilize membrane-bound proteins within a natural lipid bilayer for use in studies of protein structure analysis.
Temperature sensitive Lipodisq® can be produced by utilizing specific surfactants in place of the poly(styrene-maleic acid) chaperone molecule, whereby the surfactant fulfills a similar role to the poly(styrene-maleic acid) by interacting with phospholipid. The association of surfactant and phospholipid is again dependent upon the existence of a particular HLB within the surfactant structure, where surfactants of high chain length, >16, are generally unable to arrange their structure into the optimal shape required to trigger an association with phospholipids. Polysorbate 20 (Tween 20) (Polyoxyethylene sorbitan monolaurate) (figure 3) is one such chaperone molecule that is able to associate with phospholipid to produce a Lipodisq® complex.
|
DLS is a technique suitable for characterizing the size of nanoparticle dispersions and molecular solutions. The technique analyses the time-dependent fluctuations in the intensity of scattered light from a suspension of particles or molecules undergoing random, Brownian motion to determine their diffusion coefficients and hence particle sizes [3-6].
This application note summarizes how DLS was used to investigate the effect of changing environmental conditions (i.e. pH and temperature) upon Lipodisq® structure and particle size.
DLS measurements were made on a Malvern Zetasizer Nano S with a detection angle of 173° using a 4mW He-Ne laser operating at a wavelength of 633nm. The reported intensity weighted mean diameters in this study were determined from the cumulants analysis as defined in the International Standard ISO22412.
The effect of pH was studied using the Zetasizer Nano in combination with a Multi Purpose Titrator (MPT-2) which automatically adjusted the pH using 0.1M HCl and 0.1M NaOH as titrants. The effect of temperature on the particle size was automatically studied using the Zetasizer Nano's built in Peltier temperature control. Measurements were made at temperatures between 20 and 90°C respectively with a temperature increment of 1°C and an equilibration time at each temperature of 1 minute to ensure thermal equilibrium. Measurements from 90 to 20°C were made at 10° increments with an equilibration time at each temperature of 4 minutes.
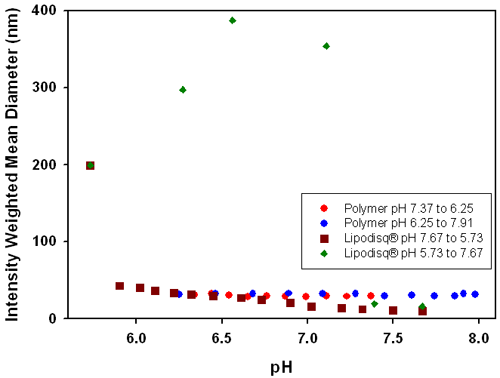
Figure 4 shows the influence of pH on the intensity weighted mean diameters for (1) a poly(styrene-maleic acid) Lipodisq® suspension and (2) for a polymer suspension without the presence of phospholipid. The plot shows that the polymer solution remains unresponsive with a diameter of around 30nm across the pH range. However, the results from the Lipodisq® suspension show a pH dependent change in size, suggesting that profound conformational changes in the structure of the polymer must occur upon association with phospholipid during formation of an assembled structure. Binding of the polymer to the phospholipid results in the adoption of a collapsed polymer structure which is highly responsive to pH changes and acts as a molecular trigger to dimensional changes of the complex. This effect is observed as the intensity weighted mean diameter of the Lipodisq® solution increases from ~12nm to more than 200nm as the pH is reduced. Subsequent increases in pH from pH 5.7 to 7.7 results in the reformation of Lipodisq® particles, as demonstrated by a gradual reduction of particle size, illustrating the versatility of this system to reform Lipodisq® particles.
|
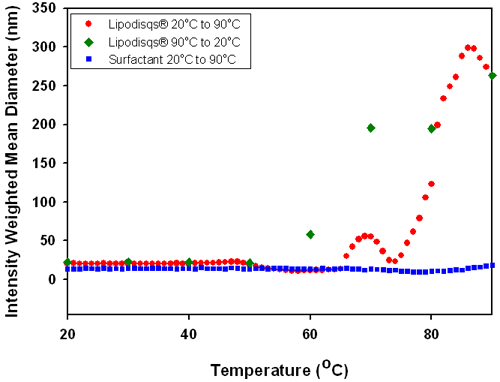
Polysorbate Lipodisq® suspensions are sensitive to temperature. With increasing temperature, the hydration of hydrophilic (particularly polyoxyethylene) groups decreases and the emulsifying agent becomes more hydrophobic - i.e. its HLB decreases [6]. The point at which this occurs is known as the cloud point. At this critical temperature the surfactant-phospholipid interaction is weakened and the complex begins to disassociate. As the temperature is increased further this disassociation will increase until the complex breaks down and releases the bound phospholipid. This observationcan be expected to occur around 76°C, the recognized cloud point of Polysorbate 20. Figure 5 shows the temperature dependence of the mean diameter of polysorbate Lipodisq® suspension and polysorbate surfactant solution.
The data in Figure 5 shows that a gradual increase in temperature of the polysorbate Lipodisq® suspension results in an increase in particle size. Disassociation of the polysorbate Lipodisq® components leads to a disruption of the complex and release of bound phospholipid to form a dense emulsion as temperatures are increased above the cloud point of polysorbate 20 (76°C). This is demonstrated by an increase in the intensity weighted mean diameter from <30nm at 50°C to approximately 300nm by 85°C. Subsequent cooling of the solution results in rapid reformation of the polysorbate Lipodisq® complex (as the HLB of the surfactant returns to its optimum range for Lipodisq® formation) with a return to the original particle size at 50°C.
|
Poly(styrene-maleic acid) Lipodisq® particles have been shown to be sensitive to changing pH conditions, with an increase in particle size from ~12nm to >200nm over a pH range of 7.7 to 5.7.
Polysorbate 20 Lipodisq® particles have demonstrated sensitivity to temperature change, increasing in particle size from ~20nm to ~300nm over a temperature range of 20°C to 90°C. Both complexes were found to spontaneously re-assemble upon reversal of conditions.
Dynamic light scattering is a non-invasive technique that can be used to study the small changes in particle size of Lipodisq® systems induced by pH or temperature changes.
[1] Compositions Comprising a Lipid and Copolymer of Styrene and Maleic Acid (2006) Patent WO 2006/129127
[2] Compositions Comprising Macromolecular Assemblies of Lipid and Surfactant (2008) Patent WO 2008/065451.
[3] B.E. Dahneke (1983), Measurement of Suspended Particles by Quasi-Elastic Light Scattering. Wiley, New York.
[4] R. Pecora (1985), Dynamic Light Scattering: Applications of Photon Correlation Spectroscopy. Plenum Press, New York.
[5] International Standard ISO22412:2008,, Particle Size Analysis - Dynamic Light Scattering, (DLS) International Organization for Standardization
[6] K. Tsujii (1998), Surface Activity: Principles, Phenomena and Applications. Academic Press, San Diego.