Prof. Hatakeyama, Dept. Eng, Nagasaki University, Prof. Nakagawa, Dr. Ogasahara, Inst. For Protein Research, Osaka University
Many biological processes rely on protein-carbohydrate interactions. A sugar binding protein extensively used for studying these interactions is Concanavalin A (Con A). This sugar binding protein structure exists as a tetramer with a molecular weight of 104 kDa. It consists almost entirely of β-sheet and is shown in figure 1.
|
The plant protein Con A was extracted from the jackbean and purified at pH 7 where a tetrameric form is expected. Samples were prepared at 1 mg/mL and 10mM of 4 mono-saccharides: Galactose, Glucose, Fructose, and Mannose. All samples were filtered with 20nm pore size Whatman Anodisk membranes and then measured in a Zetasizer Nano S.
Dynamic light scattering (DLS) measurements were performed in automatic mode and as a temperature scan from 33°C to 50°C at 0.4°C increments. Correlation functions and scattering intensity were recorded.
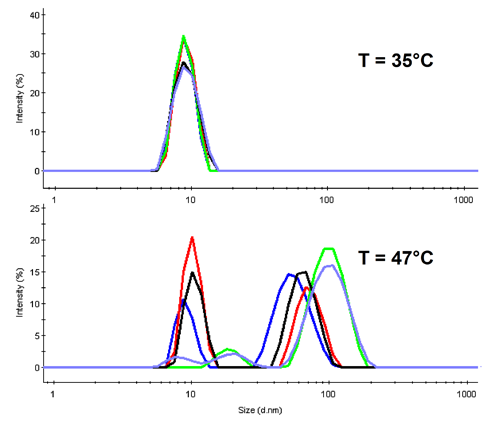
In DLS the diffusion of molecules is detected to determine their hydrodynamic size. Con A is expected to denature around 40°C, thus at lower temperatures the tetrameric protein should be present. This is confirmed with the overlay of all distribution functions at 35°C in the upper part of figure 2.
|
For temperatures above the denaturation point, we observe aggregation in all samples (lower part of fig 2 where the distributions are shown for 47°C). It is of worth noting the height of the original protein peak. Only three of the samples show a significant peak near the native size, and these are in the order Mannose > Fructose > Glucose.
The Malvern Protein Wizard report contained in the Zetasizer Nano software can be used to determine the size of the most prevalent species by mass, and average the size for all data taken from 33-41°C. The averaged peak sizes are listed in table 1. The overall average size is 8.34nm. This corresponds to an estimated molecular weight of 95 kDa using an empirical relationship between size and MW for globular proteins - clearly supporting the presence of a tetramer.
| Sample | Size [d.nm] | Standard deviation |
|---|---|---|
| Con A | 8.30 | 0.84 |
| + Galactose | 8.12 | 0.72 |
| + Glucose | 8.33 | 0.43 |
| + Fructose | 8.46 | 0.61 |
| + Mannose | 8.47 | 1.44 |
| Average: | 8.34 | 0.81 |
The observed sizes, albeit all quite close, are ordered according to Mannose > Fructose > Glucose ~ ConA > Galactose.
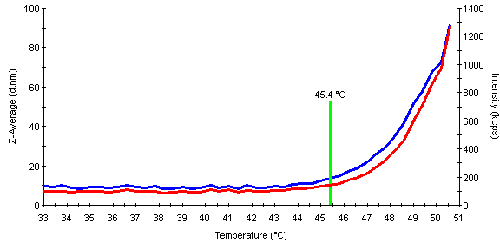
The aggregation or denaturation point is the temperature where the protein starts to aggregate significantly as a result of unfolding. The subsequent aggregation leads to an increase in the scattering intensity. This information can be used to automatically determine the aggregation point for the samples. An example of the intensity and size traces is shown in fig. 3 and values for the melting point temperature (inflection point, determined by the software) are listed in table 2. The following order for the melting points was determined: Mannose > Fructose > Glucose > Galactose > Con A.
|
| Sample | TA [°C] | Sugar bound? |
|---|---|---|
| Con A | 42.2 | No |
| + Galactose | 42.6 | No |
| + Glucose | 44.6 | Yes |
| + Fructose | 45.4 | Yes |
| + Mannose | 45.8 | Yes |
Certain sugars are expected to bind to Con A. From the measured size it appears as if Mannose binds most strongly, and possibly in higher proportion than the others. Galactose shows only very weak binding. The bound sugar protects the binding sites of the protein against unfolding, and the results obtained suggest that the Mannose complex is most stable against thermal denaturation. The observed behavior is in good agreement with expectations for these protein-carbohydrate complexes.
A sugar-binding protein was presented to various monosaccharides in solution. From the measured size and aggregation points a binding affinity series of the sugars was determined. There is some indication that the strongest binding might be due to a higher number of bound sugars per protein molecule.