S. A. Jones, G. P. Martin, B. Forbes, M. B. Brown, MedPharm Ltd, Franklin Wilkins Building, London
Several commercially available drug delivery systems, including metered dose inhalers and topical lotions, use apolar solvent systems as vehicles in the formulation of therapeutic agents. Water and oxygen that can commonly induce chemical degradation through hydrolysis, have a very low solubility in apolar vehicles and therefore, suspending particles in these systems can be a very effective method of maximizing long-term chemical stability of sensitive therapeutic agents.
The development of suspension-based formulations is typically more problematic when compared with simple solutions, as a high degree of physically stability must be maintained to ensure an accurately delivered dose throughout the shelf-life of the product. If the raw drug alone aggregates or dissolves over time, additional surfactants or stabilizing excipients are required. Typically, in apolar vehicles, it is thought that polymers and surface active surfactants stabilize suspensions as a consequence of steric hindrance i.e. parts of the solubilized excipient adsorb to the particle surface and the remaining molecular chain extends into the solvent. The interaction of two stabilizing excipient chains is energetically unfavorable and therefore the adsorbed excipient coat provides a barrier to particle-particle interaction (1). Electrokinetic stabilization of suspensions described by the Derjaguin, Verway, Landau and Overbeek (DVLO) theory is thought to have very little influence on dispersions within apolar vehicles due to the low dielectric constants of these solvents. However, a number of groups have reported suspension stabilization using excipients that have little or no solubility within such solvents (2; 3). As the stabilization of suspensions by excipients through steric hindrance requires chain extension into the continuous phase of the system, a degree of solubility in the solvent would be expected. The excipients described by Wright and Beausang et al therefore, must be using another mechanism of stabilization. The aim of this study was to establish if zeta potential influences the stability of suspensions within apolar solvents. To assess this a hydrophobic steroid was coated with four stabilizing polymers and suspended within a series of aploar solvents.
The individual polymers were adsorbed onto the surface of the steroid by spray drying the material from an aqueous suspension on a 191-lab scale machine (Buchi, Switzerland). The polymers ranged from water-soluble (polymer 1) to highly hydrophobic (polymer 4). Each of the polymers were known to have little or no solubility in the apolar solvents, the latter being chosen to represent a range of dielectric constants (ε) and partition coefficients (log P#); methyltrifluroacetate, MTA (ε 11.64, log P 1.10, RI 1.2907, η 0.418 cP ) , perfluoropentane, PF (ε 1.5, log P 3.45, RI 1.2383, h 0.462 cP &), dichloromethane, DM (ε 9.1, log P 1.01, RI 1.4229, η 0.440 cP).
The Zetasizer Nano fitted with a universal dip cell was used to measure the zeta potential (Huckel conversion used). Thermogravimetric (Mettler Toldo, UK) analysis was used to determine the water content of the polymer coated particles (n=3). Particle size and stability of the suspensions was assessed using the Mastersizer X laser diffraction particle size analyzer. The size of the dry powders was determined using a stirred cell method (1% span 80, cyclohexane mixture) and this was compared to that of the particles suspended within each of the apolar solvents. A stable suspension was defined as one which showed <20 % change in the mean volume diameter (Dv, 50) of the material over a period of 15 minutes of high-powered stirring. Statistical comparisons were performed using ANOVA on Minitab (Minitab Inc., USA).
Several of the suspensions using MTA and DCM produced a zeta potential over 40mV, which was surprisingly high for an apolar system. The absorption of polymers 1, 2 and 3 did not confer significantly different (p > 0.05‡) zeta potentials within MTA. However, a coating of polymer 4 did produce a significantly lower zeta value (p < 0.05) in this solvent. Each of the suspension systems in DCM produced a significantly different zeta potential (p < 0.05), but absorbed polymer 1 was by far the highest in the whole study at 100mV. In PF, the solvent having the lowest ε, particles showed low electrokinetic potentials.
Nevertheless, the zeta potential for each of the suspensions in PF was significantly different from each other (p < 0.05), figure 1. There was no direct correlation between zeta potential and suspension stability, which is highlighted with MTA, where three suspensions gave a high zeta potential but only one system, met the studies definition of stability. The two suspensions that were stable however, did both have a high zeta potential, see table 1.
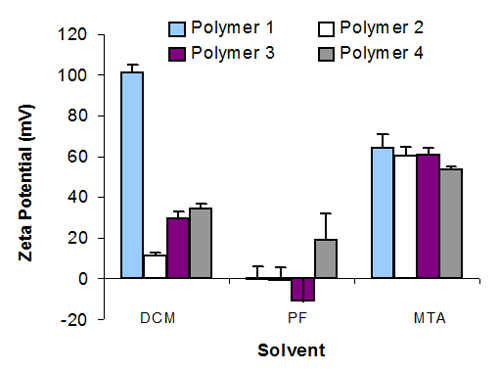
|
| Sample | Dv, 50 MTA | Dv, 50 PF | Dv, 50 DCM |
|---|---|---|---|
| Polymer 1
Polymer 2 Polymer 3 Polymer 4 | 2.92(0.20)
3.28(0.04) 2.09(0.00) 43.11(1.67) | 20.71(2.61)
32.01(6.44) 33.49(3.05) 24.13(3.07) | 3.43(0.16)
4.79(0.23) 5.29(1.98) 2.84(0.15) |
In contrast, all the PF suspensions that had a low zeta potential aggregated regardless of polymer type. Particles in the PF systems had mean particle sizes of >20 µm, (table 1). This would seem to suggest that electrokinetics does have a role to play in suspension stability, although it may not be the only important factor.
The water content of the steroid alone was 3.00 ± 0.15%. The formulations had a water content of 2.93 ± 0.15% (polymer 1), 1.57 ± 0.03% (polymer 2), 2.70 ± 0.07% (polymer 3), 0.89 ± 0.02% (polymer 4). Therefore, the process of spray drying which usually minimizes water content of formulations did not significantly alter using polymer coating 1 (p >0.05). However, applying coatings of polymers 2, 3 and 4 did significantly reduce the water contents of the steroid formulations (p < 0.05).
Suspending the polymer-coated particles in a series of apolar solvents highlighted the importance of both the solvent and polymer properties in achieving a stable suspension. Both hydrophobicity and dielectric constant appeared to influence suspension stability. Although there was not a direct correlation between zeta potential and physical stability as defined in the study, a high electrokinetic potential was associated with the two suspension systems that remained stable. This suggests that although the zeta potential of these systems may be an important factor it is not the only variable to consider in this apolar environment.
The polymer coated particles produced zeta potentials up to 102mV, which in aqueous solvents, is capable of stabilizing suspensions. The high zeta values may be due to the charging of the particles during manufacture i.e. triboelectrification as described by Cassidy et al (4). However, the influence of water in this system could also be important. Yu et al (5) elegantly showed the effect of water on zeta potential using a series of homologous solvents. Simple addition of 0.5% water to an apolar system provided one order of magnitude rise in zeta potential. This group also linked a decrease in the magnitude of zeta potential within a suspension systems to a decrease in ε (5). Results in the present study appeared to support both these observations. PF, which had a low ε produced very low electrokinetic potentials. However, MTA which had the highest ε did show the highest electrokinetic potentials across the range of polymer particles. The unique aspect of the particle suspensions used in this system compared to Yu et al, and Kitahara et al (6), who both showed the effect of water on zeta potential, is that all the water in this system is probably located at the particle-solvent interface. The hydrophobic core i.e the steroid in these microparticles is far less likely to bind the particles water compared to the polymer coat. This may induce a shift of water molecules to within the polymer and may concentrate the potential charge. The majority of the ionic species are within the system at the polymer-solvent interface therefore producing a degree of electrostatic charge.
This study indicates that electrostatic repulsion may influence suspension physical stability in solvents with a dielectric constant as low as 9.0. This mechanism could be utilized to help stabilize dispersions even in apolar media. Zeta potential should be measured in formulations using apolar solvents as it may have implications in its physical stability and hence dosing reproducibility.
(1) P. Bagchi. J. Colloid Interface Sci., 47:86-99 (1973).
(2) Beausang, E. L., Burns, S., and Buckton, G. Drug Delivery to the lung. XIV, 118-121. 2003. London..
(3) Wright.P. Respiratory Drug Delivery, IV:243-247 (1994).
(4) O. E. Cassidy, P. A. Carter, G. Rowley, and D. R. Merrifield. J. Pharm. Pharmacol., 52:13-17 (2000).
(5) J. C. Yu, Z. T. Jiang, H. Y. Liu, and J. Yu. J. Colloid Interface Sci., 262:97-100 (2003).
(6) A. Kitahara, K. Shuichi, and H. Yamada. J. Colloid Interface Sci., 25:490-495 (1967).
*Data obtained from Beilstein crossfire chemical information database (MDL information systems, GmbH)
&Measured using a U tube A viscometer #log P from ChemOffice (Cambridgesoft corporation)
‡ Further information on statistical testing can be found in Statistics for Analytical Chemistry by J.C. Miller and J.N. Miller (1993) Ellis Horwood Ltd, UK.