Best Practices for Isothermal Titration Calorimetry to study binding interactions – Part 1
Here are several best practices for performing traditional binding experiments with the MicroCal PEAQ-ITC, VP-ITC and ITC200 systems.
Sample requirements:
-
- By convention, the “Macromolecule” is the biomolecule in the isothermal titration calorimeters (ITC) cell, and the “ligand” is the biomolecule in the ITC syringe. These can be any molecules (protein, nucleic acid, small molecule inhibitor, metal, etc.) that interact with one another. “Reverse titrations” can also be done, with the “ligand” in the syringe.
- The “ligand” has one binding site, for purposes of data analysis to the one set of sites binding model, and other commonly used ITC data analysis models. The “macromolecule” can have any number of binding sites.
- All samples should be as pure as possible for ITC. Avoid or eliminate chemical and conformational impurities, since these can impact accurate concentration determination, and possibly be involved in or interfere with the binding interaction.
- For high-quality ITC data, both biomolecules must be matched in the desired buffer, to avoid heat changes due to a buffer mismatch.

- Buffer for ITC:
- Use a buffer that maintains the solubility and stability of the biomolecules, and include any salts, cofactors, and additives that are necessary for binding.
- As a starting point, you can use the same buffer utilized for other binding studies.
- ITC is compatible with aqueous buffers between pH2-pH12. Make sure the buffer concentration is sufficient to maintain pH in presence of to the biomolecules.
- If the buffer contains glycerol (or another viscous component), keep the glycerol concentration below 20% (v/v), to avoid bubbles in the ITC cell and injection syringe.
- If the buffer contains a surfactant or detergent, keep the surfactant concentration below its critical micelle concentration (CMC) unless you are studying micelles with ITC.
- If a reducing agent is needed, we recommend using TCEP or 2-mercaptoethanol. Dithiothreitol is not recommended.
- If a ligand needs DMSO for solubility, make sure you have the same final DMSO concentration in both binding partners. The recommended upper limit is 10% DMSO for ITC binding studies.
- If you need to use another organic solvent, check the instrument’s technical literature, or consult your regional Malvern Panalytical support team, for recommendations and the chemical compatibility of your ITC system.
Sample volumes:
- See Table 1. These are the recommended volumes needed to fill the ITC cell and syringe using recommended protocols, without the addition of bubbles.

Sample concentrations:
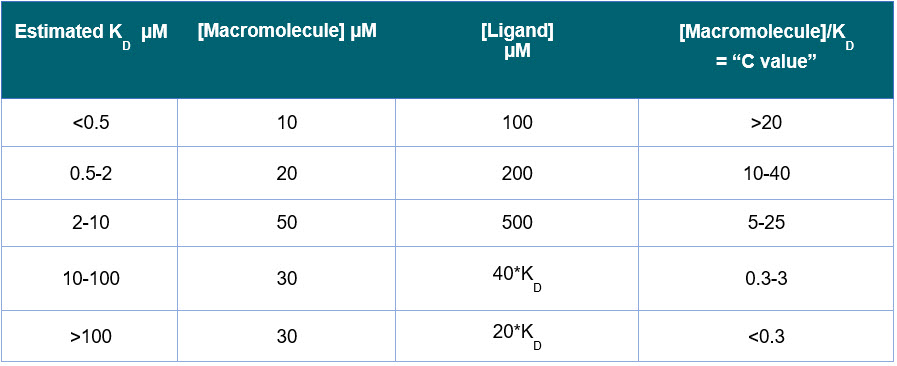
- The starting macromolecule concentration in the ITC cell is typically determined by the “C parameter” or “sigmoidocity factor”, determined by:
C= N[M]T/KD - [M]T is the macromolecule concentration in the ITC cell, and N is the stoichiometry. To get a reasonable ITC binding isotherm, the optimal range is 5 < C < 500. In many cases you can some useful data from a ITC binding curve when C is between 1 and 1000.
- For 1:1 binding, start with a 10-fold higher ligand concentration in the ITC syringe, compared to the macromolecule concentration in the ITC cell.
- If you have an estimated KD, and there is 1:1 binding, use the guide in Table 2. Assuming there is a reasonable heat change and binding enthalpy, these concentrations ranges should give a binding curve.
- If KD is unknown, try 20 μM sample in ITC cell and 200 μM sample in the ITC syringe.
- Minimum concentration in the ITC cell: 5-10 μM
- Minimum concentration in the ITC syringe: 50 μM
Relevant content:
- Isothermal Titration Calorimetry: Theory and Practice
- Practical tips for MicroCal PEAQ-ITC experiments
- Addressing complexity of binding interactions with ITC – get the most out of your ITC data
- Biomolecules: sample and data quality in interaction analysis – Two sides of the same coin
- Don’t throw away your “bad” N-value ITC data
- Gold standard for binding affinity
Previous posts:
- Best Practices for ITC to study binding interactions – Part 2
- Best Practices for ITC to study binding interactions – Part 3
- Best Practices for ITC to study binding interactions – Part 4
- Isothermal Titration Calorimetry – A hot characterization tool for biomolecular interactions
- ITC journal club
- What’s new in ITC? June 2018 edition

