MicroCal PEAQ-ITC (from Malvern Instruments) is the most recent model of ITC instrumentation, with enhanced sensitivity, improved washing module, and convenient analysis software compared to previous MicroCal ITC systems. Malvern Instruments has comprehensive manuals and videos to support the use of MicroCal PEAQ-ITC and data analysis. This Technical Note supplements these manuals, and provides additional practical tips that will contribute to improved performance of MicroCal PEAQ-ITC. Use of MicroCal PEAQ-ITC will contribute to the understanding of binding energetics and broaden the application of ITC.
Isothermal titration calorimetry (ITC) is one of the most powerful and convenient approaches to thermodynamically investigate intermolecular interactions by detecting any reaction heat that occurs in the ITC cell[1-9]. ITC offers valuable information on binding affinity, driving forces, binding stoichiometry and mechanisms, (de)hydration, (de)protonation, interactions with salts, interacting surface areas, and molecular flexibility such as protein dynamics[1-8,10-15]. The ability of ITC to follow Michaelis-Menten kinetics[16], hydrolysis[17], and estimate the exchange rates (i.e., association and dissociation rates) of molecular interactions[18] has further extended the utility of ITC to a variety of biological systems.
In the past decade, we have encountered two ITC instruments, VP-ITC (MicroCalTM, Malvern Instruments, UK) and ITC200 (MicroCalTM, Malvern Instruments, UK) that have markedly contributed to thermodynamic studies on molecular associations. PEAQ-ITC (MicroCalTM, Malvern Instruments, UK) has recently appeared as a new model of the ITC instrument with enhanced sensitivity, a more convenient washing module, and convenient analysis software[19]. Therefore, MicroCal PEAQ-ITC is also expected to contribute to our understanding of binding energetics and broaden the application of ITC.
In order to obtain accurate and precise ITC thermograms and data, a correct understanding of the procedures used for ITC experiments is required. Several comprehensive manuals on PEAQ-ITC are very useful and available[20] for obtaining information on how to successfully perform experiments and analyze data. We herein provide practical and key tips based on our experience of ITC experiments for the better performance of PEAQ-ITC and its analysis that are not addressed in these manuals.
A smaller cell volume in the PEAQ-ITC instrument (approximately 0.2 mL) than in the VP-ITC instrument (approximately 1.42 mL) is very advantageous; it reduces the amount of sample and experimental time needed and allows for the measurement of heat changes that are not clearly observed using VP-ITC, such as the heat associated with the dilution of salts at a high concentration (Figure 1). Intermolecular interactions with low affinity require ITC titration to a high molar ratio, for which large amounts of sample should be used. Fast equilibration and measurements maximize the number of ITC measurements for calculating standard deviations and the opportunities to examine various samples and conditions in a given time period. These advantages are also very effective in ITC studies using aggregation-prone samples because they minimize the time available for aggregation. Furthermore, the smaller cell volume of the PEAQ-ITC instrument is useful for reducing the potential nucleation of aggregation. However, several issues may be encountered when performing PEAQ-ITC with a smaller volume than VP-ITC, as described below.
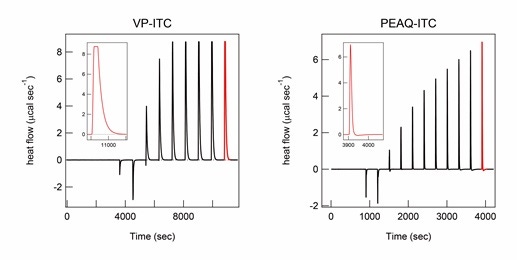
Figure 1. Heat of titration of high concentrations of NaCl monitored by different models of ITC instruments. ITC thermograms of titration of 1M NaCl in 10 M HCl solution in the ITC titration syringe to protein A in 10 M HCl solution in the ITC cell were recorded using the VP-ITC (left) and PEAQ-ITC instrument (right). ITC peaks after the final titration are shown in red and magnified in the inset for comparison. The molar concentration of NaCl after each titration of VP-ITC and PEAQ-ITC measurements was the same. The titration volume was 2 µL for the first titration and 20 µL for the other titrations using the VP-ITC, and 0.8 µL for the first titration and 2.2 µL for the other titrations using the PEAQ-ITC
Data obtained in ITC experiments with the smaller cell volume of the PEAQ-ITC instrument will be influenced more by the presence and formation of bubbles in the cell, which can create baseline spikes and low baselines, than will experiments using the VP-ITC instrument. Therefore, clearance of bubbles from the cell is routinely performed. However, users may feel that it is not easy to load a small volume of solution into the PEAQ-ITC cell using a loading syringe to exclude bubbles, particularly those who are familiar with VP-ITC, which has an approximately 7-fold larger cell volume. Strong purging (i.e., fast loading) can splatter the sample solution, leading to sample loss. However, weak purging (i.e., slow loading) will not effectively expel bubbles. Thus, pumping of solution into the cell needs to be performed as gently as possible without splattering the sample solution. Rotating the loading syringe as it is pulled out is also useful for avoiding bubbles. Additional lamps or lights help to detect bubbles, as no light is available in the cell unit.
Special care is encouraged when loading and purging colored samples, because colors, particularly at relatively high concentrations, can hamper observations of bubbles, and also when bubble-forming samples such as surfactants are used. Experiments using colored membrane proteins in a surfactant-containing solution are typical examples. A short period of centrifugation using a tabletop centrifuge and/or degassing is effective for avoiding the generation of bubbles.
Repeating the insertion and removal of a titration syringe may be the final step to ensuring a bubble-free state of sample solution in the ITC cell. If you observe noisy ITC thermograms during equilibration or the initial delay, stop the measurement and repeat the insertion and removal of a titration syringe again.
The volume of the titration syringe of the PEAQ-ITC instrument (approximately 40 µL) is also smaller than that of the VP-ITC instrument (approximately 280 µL). Therefore, bubbles remaining in the titration syringe of the PEAQ-ITC instrument may influence the accurate and precise volume of titration more than that of the VP-ITC. If bubbles are detected, click the 'Plunger/Refill' button in the instrument control bar located at the bottom of the MicroCal PEAQ-ITC control software window and attempt to remove them. A short centrifugation and/or degassing are effective for avoiding the generation of bubbles.
When bubbles persist, execute the following procedures by controlling several key functions of the ITC titration syringe in the instrument control bar:
I. Click the 'Plunger Down' button to empty the titration syringe
II. Click the 'Load' button
Note 1: The 'Load' function is suggested because it is accompanied by degassing, which is effective for removing bubbles
Note 2: 'Open Fill Port' →'Plunger Down' is often effective prior to performing the 'Load' function
III. If bubbles are not eliminated, repeat procedures I and II
IV. If the above methods do not work, refill with sample which has been subjected to a short centrifugation with a benchtop centrifuge and/or degassing. Check the plunger tip and consider replacing it after it has been used for more than 200 - 300 ITC measurements
If you are planning to use the same sample in the titration syringe for the following ITC measurement, you may fill the titration syringe with the same sample without washing or rinsing the syringe (i.e., just washing the cell). After rinsing the needle tip of the titration syringe with working solution and wiping it, click the 'Plunger Down' button and then the 'Load' button. This is an effective way to load the sample without introducing bubbles; however, caution is needed for aggregation-prone samples.
Users generally rinse the ITC cell with buffer solution, which does not contain analytes. This is a fundamental and important procedure for matching solution components between solutions in the syringe and cell, and thus minimizing background heat (i.e., control heat). However, it is difficult in practice to remove all solution remaining in the cell using the loading syringe. The effects of remaining solution in the cell of the PEAQ-ITC instrument on changes in the concentration of analytes will be larger than those of the VP-ITC instrument, because of the smaller cell volume in the former, compared to the latter.
Analyses using an incorrect sample concentration will compromise evaluations of the n-value, affinity constant (association and dissociation constants), and other thermodynamic parameters, including changes in enthalpy (∆H), entropy (∆S), and Gibbs free energy (∆G) with high accuracy and reproducibility. In order to minimize this issue, it is recommended that the cell is rinsed with working sample solution, following rinsing with analyte-free buffer solution.
In order to load the sample solution and perform subsequent pumping to remove bubbles, insertion of the loading syringe into the cell is an imperative procedure that expels sample solution corresponding to the volume of the needle of the loading syringe. If sample solution remains in a cell reservoir before removing the loading syringe, the cell will be filled with sample solution.
The systematic cleaning system in the PEAQ-ITC instrument, which largely comprises the washing module, cell unit, and controller PC, is very efficient and robust. However, sticky samples may remain in the cell reservoir and bottom of the retaining nut of a pipette. Thus, cleaning of the cell reservoir and the bottom of the retaining nut is advisable in order to ensure clean ITC thermograms and successful analyses (Figure 2).
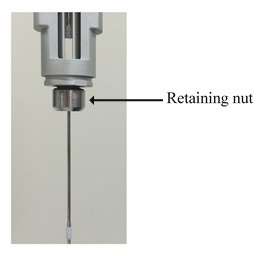
Figure 2. Illustration of the retaining nut in the PEAQ-ITC pipette unit. The retaining nut is indicated with an arrow and label
The PEAQ-ITC system is intuitive and sensitive. Thus, it senses any imperfection in the system and produces various case-sensitive messages for errors. When error messages occur regarding cleaning steps, carefully verify every connection of the fluid lines, capping states of bottles, and amounts of solution in each bottle (doubly deionized water (DDW), 14% DECON or 20% ContradTM 70, and methanol (HPLC grade or >99% pure), and fluid waste) in the washing module as well as connections between the washing module, cell unit, and controller PC.
6. 1. Recover remaining sample from the titration syringe by clicking the 'Plunger Down' button in the instrument control bar, after rinsing the tip of the titration syringe with working solution.
6. 2. Rinse the tip of the titration syringe with working solution and wipe the tip as rapidly and gently as possible to not deform the needle of the titration syringe before insertion of the ITC titration syringe into the ITC cell. Otherwise, titrants will react with analytes in advance of ITC experiments. This procedure becomes more important when the concentrations of titrants are very high.
6. 3. Before refilling the reference cell with DDW, ensure that the cell reservoir is clean, otherwise DDW in the reference cell may become polluted because DDW will overflow from the reference cell during refilling. Keeping the cell unit clean is a prerequisite of ITC.
1 Jelesarov, I. and Bosshard, H. R. (1999) Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J Mol Recognit 12, 3-18
2 Pierce, M. M., Raman, C. S. and Nall, B. T. (1999) Isothermal titration calorimetry of protein-protein interactions. Methods 19, 213-221
3 Leavitt, S. and Freire, E. (2001) Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr Opin Struct Biol 11, 560-566
4 Ladbury, J. E. and Williams, M. A. (2004) The extended interface: measuring non-local effects in biomolecular interactions. Curr Opin Struct Biol 14, 562-569
5 Velazquez Campoy, A. and Freire, E. (2005) ITC in the post-genomic era...? Priceless. Biophys Chem 115, 115-124
6 Ladbury, J. E. (2010) Calorimetry as a tool for understanding biomolecular interactions and an aid to drug design. Biochem Soc Trans 38, 888-893
7 Lee, Y. H., Ikegami, T., Standley, D. M., Sakurai, K., Hase, T. and Goto, Y. (2011) Binding energetics of ferredoxin-NADP+ reductase with ferredoxin and its relation to function. Chembiochem 12, 2062-2070
8 Kim, J. Y., Nakayama, M., Toyota, H., Kurisu, G. and Hase, T. (2016) Structural and mutational studies of an electron transfer complex of maize sulfite reductase and ferredoxin. J Biochem 160, 101-109
9 Kim, J. Y., Kinoshita, M., Kume, S., Tomas, H. G., Sugiki, T., Ladbury, J. E., Kojima, C., Ikegami, T., Kurisu, G., Goto, Y., et al. (2016) Noncovalent forces tune the electron transfer complex between ferredoxin and sulfite reductase to optimize enzymatic activity. Biochem J 473, 3837-3854
10 Markova, N. and Hallen, D. (2004) The development of a continuous isothermal titration calorimetric method for equilibrium studies. Anal Biochem 331, 77-88
11 Olsson, T. S., Williams, M. A., Pitt, W. R. and Ladbury, J. E. (2008) The thermodynamics of protein-ligand interaction and solvation: insights for ligand design. J Mol Biol 384, 1002-1017
12 Fukuhara, A., Nakajima, H., Miyamoto, Y., Inoue, K., Kume, S., Lee, Y. H., Noda, M., Uchiyama, S., Shimamoto, S., Nishimura, S., et al. (2012) Drug delivery system for poorly water-soluble compounds using lipocalin-type prostaglandin D synthase. J Control Release 159, 143-150
13 Kume, S., Lee, Y. H., Miyamoto, Y., Fukada, H., Goto, Y. and Inui, T. (2012) Systematic interaction analysis of human lipocalin-type prostaglandin D synthase with small lipophilic ligands. Biochem J 446, 279-289
14 Kume, S., Lee, Y. H., Nakatsuji, M., Teraoka, Y., Yamaguchi, K., Goto, Y. and Inui, T. (2014) Fine-tuned broad binding capability of human lipocalin-type prostaglandin D synthase for various small lipophilic ligands. FEBS Lett 588, 962-969
15 Kinoshita, M., Kim, J. Y., Kume, S., Sakakibara, Y., Sugiki, T., Kojima, C., Kurisu, G., Ikegami, T., Hase, T., Kimata-Ariga, Y., et al. (2015) Physicochemical nature of interfaces controlling ferredoxin NADP(+) reductase activity through its interprotein interactions with ferredoxin. Biochim Biophys Acta 1847, 1200-1211
16 Todd, M. J. and Gomez, J. (2001) Enzyme kinetics determined using calorimetry: a general assay for enzyme activity? Anal Biochem 296, 179-187
17 Maximova, K. and Trylska, J. (2015) Kinetics of trypsin-catalyzed hydrolysis determined by isothermal titration calorimetry. Anal Biochem 486, 24-34
18 Vander Meulen, K. A., Horowitz, S., Trievel, R. C. and Butcher, S. E. (2016) Measuring the Kinetics of Molecular Association by Isothermal Titration Calorimetry. Methods Enzymol 567, 181-213
19 Microcal PEAQ ITC Range