In this Application Note, one copolymer and one polymer mixture, both comprised of polystyrene (PS) and poly(methyl methacrylate) (PMMA), will be analyzed using GPC/SEC in order to determine the relative concentration of each monomer present. The results will be presented and comparisons will be made between the two.
Over the last century, as synthetic polymers became more prevalent, a desire arose to fine-tune the physical properties of these materials to produce the optimal end-product for a given application. One method researchers explored was to mix two or more types of monomers so that a single chain of the resulting polymer would be a combination and potentially possess a unique set of physical properties. Copolymers, the term used to describe these polymers consisting of two or more different constituent units, can exhibit a variety of monomer patterns and structures (e.g. random, alternating, block, comb). While the monomer arrangement and overall shape of the copolymers can affect their physical properties, so can the relative concentration of each monomer present. For example, in styrene-butadiene rubber, when the concentration of styrene is high the resulting rubbers are more rigid. With low styrene content, the materials are softer and more elastic. Additionally, mixing two different polymers where the individual polymer chains are not covalently bonded is an alternate, and sometimes more accessible, method to produce materials with customized properties. These non-covalently bonded samples are referred to as polymer blends or mixtures. Knowing the relative concentration of each monomer present in a copolymer or mixture is critical to understanding the physical properties of the end-product.
One analytical technique that can provide this information is gel permeation chromatography (GPC) or, equivalently, size-exclusion chromatography (SEC). GPC/SEC is a widely used technique to characterize a wide variety of macromolecules, from bulk manufactured materials to natural polymers and proteins. This technique can be used to measure the molecular weight moments (Mw, Mn), molecular weight distribution (Mw/Mn), intrinsic viscosity (IV) and hydrodynamic size (RH) of these macromolecules. Figure 1 shows Malvern’s OMNISEC, a complete, all-inclusive GPC/SEC system.
A brief overview of how GPC/SEC works: A solvated sample is carried by a liquid mobile phase through an analytical column full of porous gel particles, where diffusion-controlled separation of the macromolecular components occurs, and is ultimately observed by different detectors as each slice of sample elutes. A common advanced detection GPC/SEC setup includes refractive index (RI) UV/photodiode array (PDA) detector, viscometer, and light scattering detectors. For reasons that will be explained below, a minimum configuration of RI and UV/PDA detectors is required for copolymer / compositional analysis.
In this Application Note, one copolymer and one polymer mixture, both comprised of polystyrene (PS) and poly(methyl methacrylate) (PMMA), will be analyzed using GPC/SEC in order to determine the relative concentration of each monomer present. The results will be presented and comparisons will be made between the two.

Figure 1: Malvern’s OMNISEC Tetra Detection GPC/SEC System
Malvern’s copolymer or compositional analysis method is designed to determine concentration information for materials comprised of two distinct monomers. In order for the copolymer / compositional analysis method to work well, a few conditions must be met. Both monomers must have measurable refractive indices in the mobile phase and both of the dn/dc values (or refractive index increment values) must be known. The two monomers must possess different absorbance profiles; ideally one monomer will absorb at a given wavelength of light and the other one won’t. If there is no specific wavelength where one monomer absorbs and the other does not, the relative absorption at a given wavelength must be known (alternately, one can use the dA/dc values of the individual components). By using the data from two concentration detectors the software can set up two calculations (equations [1] and [2]) to solve for the two unknowns (concentrations of A (CA) and B (CB)). This is why it is imperative to know the dn/dc and dA/dc values for each component of the copolymer; without them there is no way to know how much of the total RI or UV signals to attribute to each monomer.
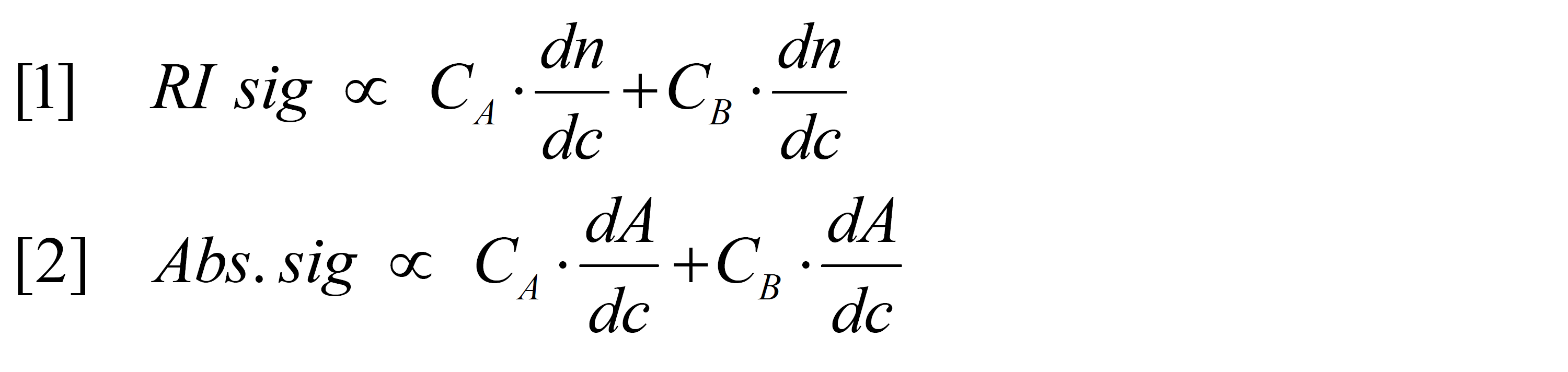
It is important to remember that the copolymer / compositional analysis method does not reveal the chromatograms of individual components in a sample, but provides the concentration of each component in a sample. These concentration plots may look similar to the RI or UV chromatograms because those detectors respond to sample concentration, however they cannot be analyzed further as a chromatogram. The analysis operates the same regardless if the components are covalently bonded, as in a copolymer, or simply mixed together.
The PS/PMMA copolymer was analyzed in a mobile phase of THF using 3 × Malvern LT-3000L columns. The sample solution was prepared at a concentration of 2.8 mg/mL in THF and the injection volume was 100 µL. The tetra detector chromatogram for the copolymer sample is shown in Figure 2. The RI signal is presented in red, the UV signal from the PDA is purple, the viscometer signal is blue, the right angle light scattering detector is green and the low and light scattering detector is black. Limits of integration and baseline points are also shown.
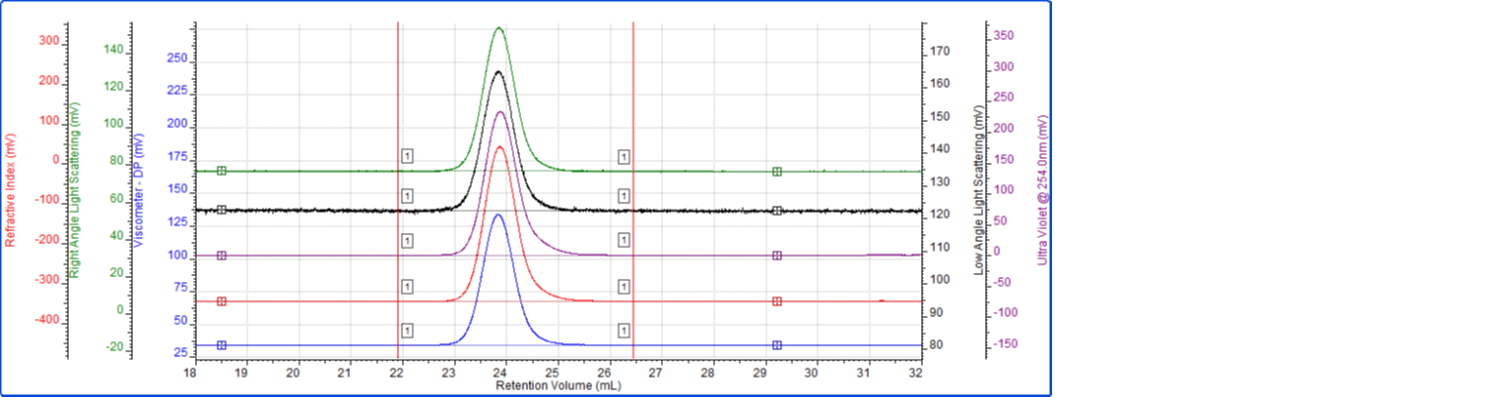
Figure 2: Tetra detector chromatogram of the copolymer sample
In setting up the copolymer / compositional analysis method the dn/dc value used for PS in THF was 0.185 and for PMMA in THF was 0.085. The dA/dc values used for PS and PMMA were 1 and 0, respectively. These are not the absolute dA/dc values for PS and PMMA, but since PS has an absorption at 254 nm and PMMA doesn’t, then all of the UV absorption data will be attributable to PS making the all or nothing values of 1 and 0 appropriate.
The molecular characterization data for the copolymer sample is shown below in Table 1. In addition to the Mw, Mn, Mw/Mn, IV and RH, the weight fractions of PS and PMMA are listed. Data for three injections are presented, along with relative standard deviations.
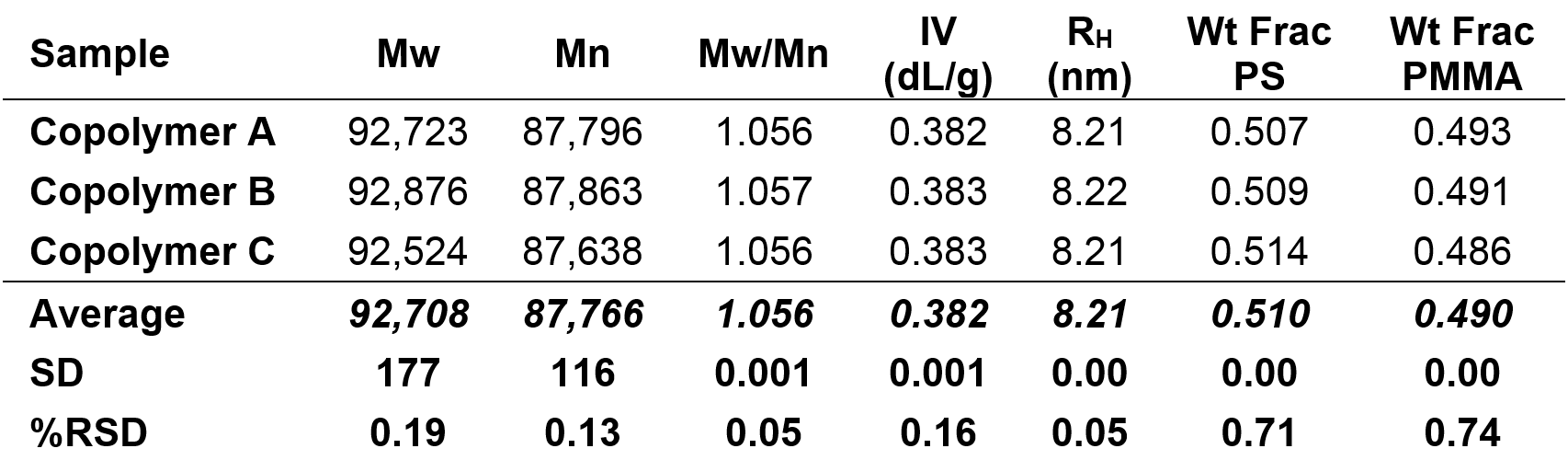
Table 1. Molecular characterization data for three injections of the copolymer sample; Mz, Mw, Mn in Da
The data for the copolymer sample is consistent with the chromatograms shown in Figure 2, representing a well-defined sample with a narrow distribution. It is important to note that this copolymer sample has a single distribution, meaning the peak is not bimodal and there is only one sample peak present. This indicates that the two different monomers exist within this single distribution. This is a common characteristic of copolymers; however, this is not absolutely true for all examples of copolymers.
The weight fraction data provides insight into the relative concentration of each monomer. The weight fraction of PS was found to be 0.51 and the weight fraction of PMMA was found to be 0.49. When the molecular mass of each monomer is taken into account (104.15 Da for styrene and 100.121 Da for methyl methacrylate), the weight fractions of PS and PMMA indicate that the monomers exist in a 1:1 ratio (a 1:1 copolymer would have weight fractions of 0.51 for PS and 0.49 for PMMA from PS = 104.15 Da/204.271 Da; PMMA = 100.121 Da/204.271 Da).
The weight fraction provides relative concentration information, but doesn’t provide insight into how that concentration exists over the MW range of the sample. In order to determine if the two components exist evenly throughout the material or if one monomer is biased to the high or low MW material, a visual representation of the individual monomer concentrations, as shown in Figure 3, is examined.
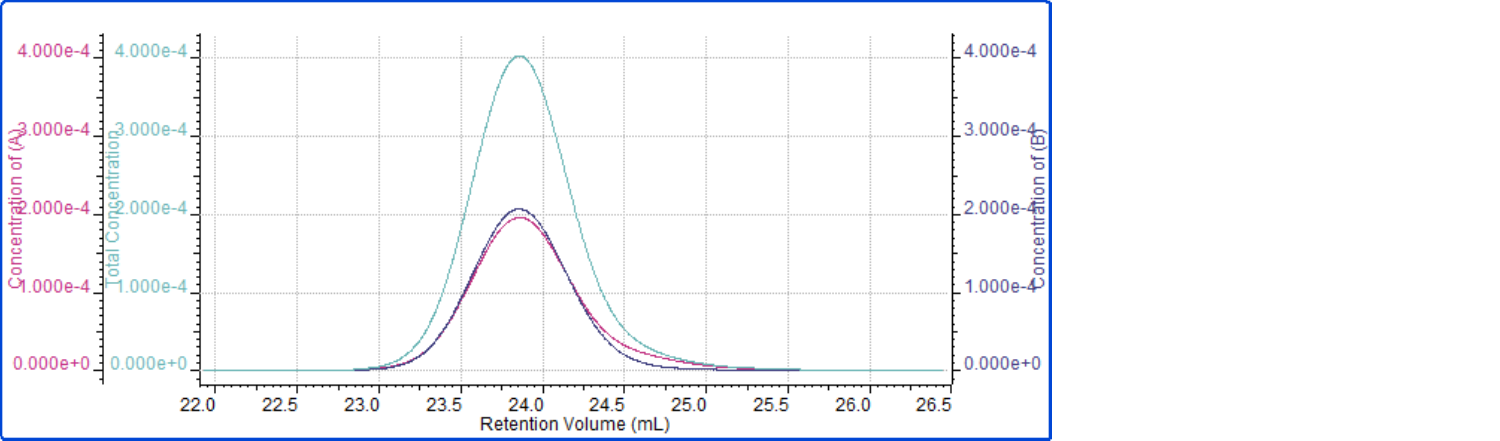
Figure 3: Concentrations of PS (magenta), PMMA (dark blue), and total sample (aqua) plotted against retention volume for the copolymer sample
The concentration plots in Figure 3 indicate that the concentrations of PS and PMMA are very similar at each point throughout the sample’s molecular weight distribution. Regardless of the molecular weight of a polymer chain within this sample, the ratio of PS to PMMA will be about 1:1. This type of information can assist researchers in determining how effectively their copolymerization processes are working and if the end-product displays the desired characteristics.
To complement the analysis of the copolymer sample, a polymer mixture sample was analyzed. The analysis conditions were similar, using a mobile phase of THF and 2 × Malvern T6000M columns. The sample solution was prepared at a concentration of 2.5 mg/mL in THF and the injection volume was 100 µL. The tetra detector chromatogram for the polymer mixture sample is shown in Figure 4. As above, the RI signal is presented in red, the UV signal from the PDA is purple, the viscometer signal is blue, the right angle light scattering detector is green and the low and light scattering detector is black. Limits of integration and baseline points are also shown.
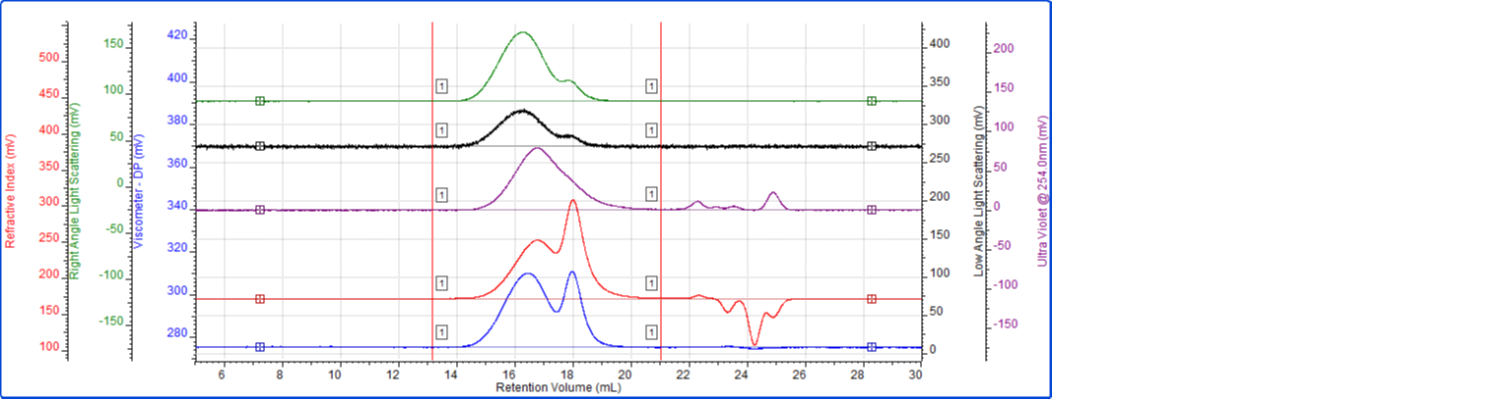
Figure 4: Tetra detector chromatogram of the polymer mixture sample
As with the previous example for the copolymer sample, the dn/dc value used for PS in THF was 0.185 and for PMMA in THF was 0.085. The dA/dc values used for PS and PMMA were 1 and 0, respectively.
The molecular characterization data for the polymer mixture sample is shown below in Table 2. In addition to Mw, Mn, Mw/Mn, IV, and RH, the weight fractions of PS and PMMA are listed. Data for three injections are presented, along with relative standard deviations.
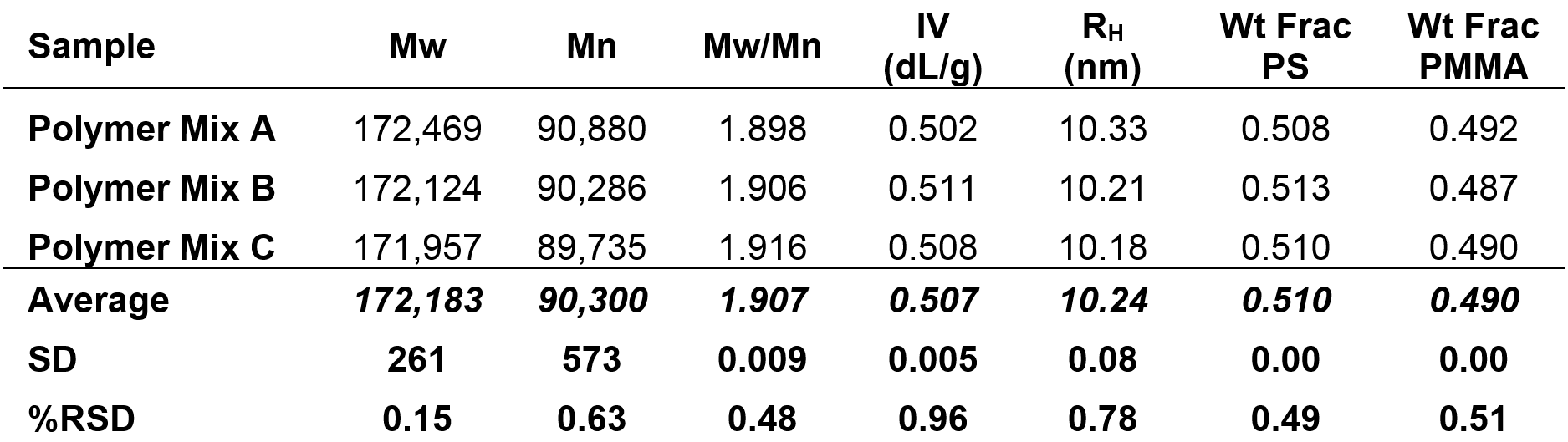
Table 2. Molecular characterization data for three injections of the copolymer sample; Mz, Mw, Mn in Da
The data for the polymer mixture provides reproducible average values for the entire bimodal sample distribution. As opposed to the copolymer sample analyzed above, the polymer mixture clearly shows two peaks in most of the detector responses shown in Figure 4. While this does not necessarily indicate that they are composed of different types/ratios of materials, the differences in the detector responses of each peak suggest that to be the case. Specifically, a comparison of the RI and UV chromatograms for the polymer mixture reveals that the narrower peak eluting at 18 mL is present in the RI signal but not the UV detector response. This means that whatever material is responsible for that peak does not have a UV absorbance and is different than the material eluting earlier that provides the broad peak in the UV signal (14-20 mL).
As with the copolymer sample, the weight fraction data provides a thorough analysis of the relative concentration of each monomer. The weight fraction of PS in the polymer mixture was found to be 0.51 and the weight fraction of PMMA was found to be 0.49. Like the copolymer example previously explored, these weight fractions indicate a 1:1 ratio of PS to PMMA in the polymer mixture sample.
So while the weight fractions indicate the PS:PMMA ratio in the polymer mixture is 1:1, the weight fractions do not specify if the monomers are evenly distributed in a manner similar to the copolymer example. By viewing the concentrations of each component as a function of elution volume, as shown in Figure 5, it can be seen that the two components mostly comprise different portions of the chromatograms. This makes sense for a sample that is simply a mixture of two polymers, since each component would have its own distribution that may or may not overlap with the other.
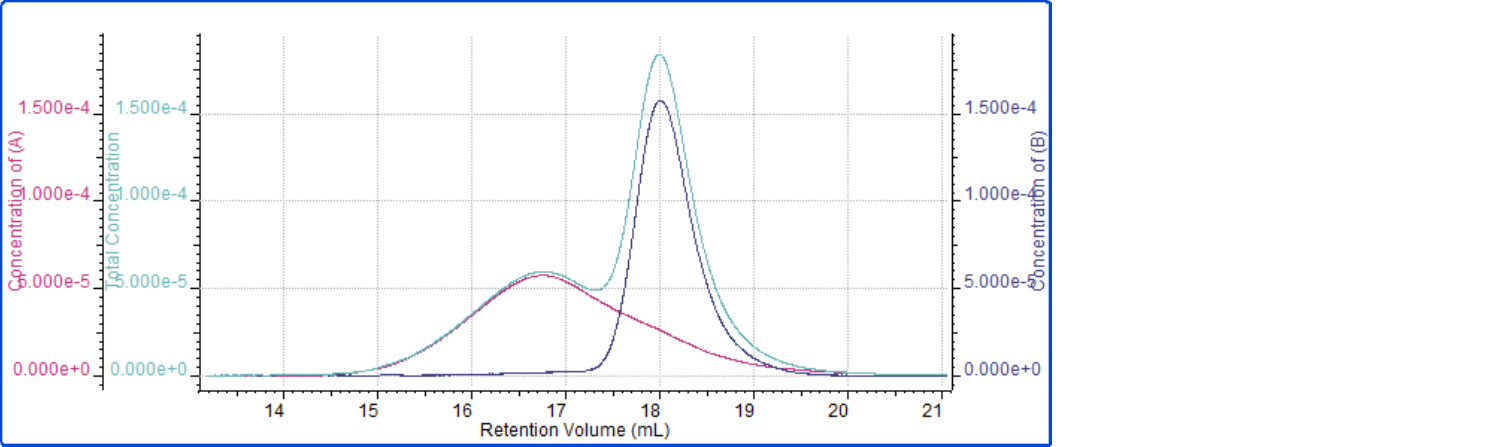
Figure 5: Concentrations of PS (magenta), PMMA (dark blue), and total sample (aqua) plotted against retention volume for the polymer mixture sample
The concentration plots in Figure 5 reveal that PS and PMMA in the sample possess separate distributions within the mixture. The earlier eluting, broader peak is pure PS, while the later eluting, narrower peak is mostly PMMA, with some low molecular weight material from the PS distribution contributing. This is in contrast to the evenly distributed styrene and methyl methacrylate components in the copolymer sample previously discussed. Both samples are comprised of PS and PMMA in a 1:1 ratio, however the way the two monomers are distributed throughout the samples are vastly different. Observation of this difference is only accessible when using a GPC/SEC setup that includes both an RI and UV detector.
Malvern’s OMNISEC tetra detection GPC/SEC system provides outstanding chromatography data for the analysis of both the copolymer and polymer mixture samples. The analyses revealed reproducible molecular characterization data and included the weight fractions of each component within the samples. Both samples were found to contain a PS:PMMA ratio of 1:1. While their chemical constituency was identical, the MW profiles of the samples were found to be quite different. The different MW profiles observed supported the notion that the two samples were different types; one a copolymer with a single MW distribution and one a mixture of two polymers, each with their own distribution. The copolymer / compositional analysis method provided accurate data regardless of the physical relationship between the PS and PMMA within each sample.
It should be noted that the same analysis techniques described here are not limited to synthetic polymers and can be applied to other application areas. The copolymer / compositional analysis method has been beneficial in the life-science arena, specifically to study protein-PEG conjugates or membrane proteins in detergent micelles.
The ability to test and measure the concentrations of components within a sample as part of advanced GPC/SEC analysis offers a valuable set of data and results. This type of sophisticated analysis can provide researchers and manufacturers the insight needed in order to develop and produce unique products for specific applications.