Proteins may be stabilized both by their local buffer solvent environment and by internal bonds. Metal ion specific and non-specific binding is a key influence on the structural integrity of many proteins. The interactions of a protein with certain metal ions may result in pronounced structural changes, with such conformational alterations hypothesized to promote the pathogenicity and virulence of certain infectious agents, and regulate cytotoxicity in prion protein[1].
The primary investigation detailed in this application note compares the structural stability of carbonic anhydrase (CBA) in solution, with and without excess metal ions within the solvent, across a temperature gradient and at different pH values. The z-average size (radius in nm) and electrophoretic mobility (µmcm/Vs) were automatically measured across a temperature range of 20ºC to 70ºC for each sample, and the data sets analyzed.
All measurements were acquired using the new Zetasizer Nano ZSP. The diffusion barrier method, described in application note MRK1651-02, was used during acquisition of zeta potential measurements in order to prevent instrument-induced degradation of the samples.
This note highlights the benefits of an orthogonal approach to measurements of protein structural stability.
|
All samples were prepared at a concentration of 10 mg/mL in 10 mM HEPES buffer (Sigma-Aldrich, Poole, UK), which was diluted from 10x stock with Fresenius sterile water (Fresenius Kabi, UK). The buffer was then equally split into two sterile 500 mL bottles and zinc acetate (Sigma Aldrich, Poole, UK) was added to a final concentration of 5 mM to one of the bottles of buffer. All buffers were pH-adjusted with 1 M sodium hydroxide or 1 M hydrochloric acid and vacuum sterile filtered to 0.02 µm. Protein samples were prepared in the appropriate buffer and again sterile-syringe filtered to 0.02 µm (Fisher Scientific, 50-822-149). The diffusion barrier method was used for the zeta potential measurements of the proteins unless otherwise specified, to prevent protein degradation and denaturation. Duplicate data sets were collected and the average values plotted, with the plot inflection point taken as the point of aggregation/denaturation. The instrument was set up with default parameters, unless otherwise specified in the Appendix: Tables 1 & 2.
Bovine carbonic anhydrase (CBA) catalyzes the reaction of carbon dioxide and water to produce carbonic acid and hydrogen.
[(-hys)3Zn(H2O)]2+ + CO2 → [(-hys)3Zn]2+ + HCO3- + H+
Hydration is inhibited by imidazole and dehydration is inhibited by aromatic or heterocyclic sulphonamides. Zinc is required for carbonic anhydrase to adopt an active conformation, a zinc ion being bound to three histidyl residues (His 94, His 96 and His 119) in the active enzyme (see figure 1). The potent inhibitor acetazolamide (2-acetylamino-1,3,4-thiadiazole-5-sulphonamide) is unable to bind to the catalytic site in the zinc-free isoform[2].
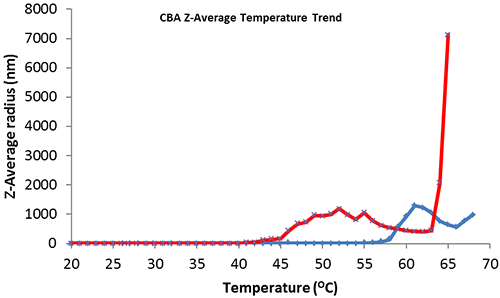
Thermal trend monitoring of the average size of CBA (figure 2, blue) shows the point of heat denaturation or aggregation onset at 58ºC which corresponds with the published melting point (Tm) value (carbonic anhydrase II - 25 mM PIPES, 100 mM NaCl, 0.5 mM EDTA at pH 7.0)[3]. CBA with excess addition of 5mM ZnAc (figure 2, red) shows a significant reduction in thermal protein structural stability with aggregation onset at 40ºC.
|
|
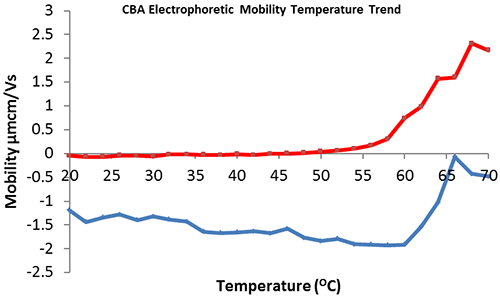
The effect of temperature on the electrophoretic mobility of CBA in HEPES buffer in the presence and absence of 5mM zinc acetate was investigated. The collected data (figure 3) demonstrates that CBA in the absence of zinc has an electrophoretic mobility of -1.5 µmcm/Vs (figure 3, blue) that is reduced in magnitude at temperatures greater than or equal to 60ºC, suggesting the onset of protein denaturation/aggregation. This supports the comparative size/temperature trend (figure 2, blue) which illustrates aggregation onset at 58ºC. Addition of 5 mM zinc acetate to the buffer reduces the electrophoretic mobility of CBA, with a point of positive inflection at approximately 48ºC, indicating that protein denaturation/aggregation begins at this temperature. This data supports the comparative size/temperature trend (figure 2, red).
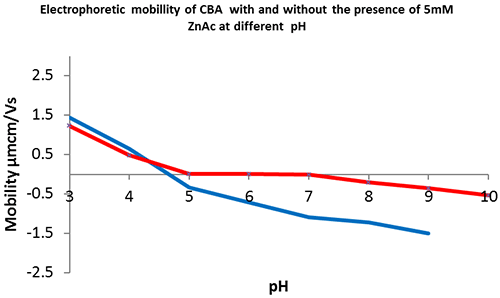
The reduced stability of CBA-Zn and lack of global charge over the pH range 5 to 7 suggests that zinc is involved in, and disrupts, intermolecular interactions.
|
The kinetic reactivity of CBA is dependent on a solvent pH of between 5 and 8, with the maximum observed rate of reaction at pH 8. The mid-point for activity is pH 7, suggesting that the catalytically active group has a pKa of 7 and that the high pH deprotonated isoform is more catalytically active. This would support the hypothesis: that it is a zinc-bound hydroxide ion that drives this simple mechanism for hydration of carbon dioxide[4].
CBA is only catalytically active at pH 5 to 8 with a bound zinc ion at its active site. The data collected shows how the protein's electrophoretic mobility varies at different pH values, with and without excess free zinc in the buffer. The CBA without zinc (figure 4, blue) has no overall charge at pH 4.5. CBA in the presence of excess zinc (figure 4, red) remains near neutral between pH 5 and 8, the pH range within which the enzyme is known to be in its active conformation and capable of substrate catalysis. In the previous experiments (figures 2 & 3) it was demonstrated that CBA in the presence of excess free zinc has a significantly lower (greater than or equal to 48ºC) melting temperature than CBA in the absence of zinc (greater than or equal to 60ºC). Therefore by comparing its electrophoretic mobility at different pH values, we can elucidate how a particular formulation may influence the overall charge of certain proteins and further understand the solution state of drug candidates or proteins of interest. In conclusion, the Zetasizer Nano ZSP provides an invaluable tool for assessing protein structural stability with two orthogonal approaches within one system.
| Type | Parameter | Setting |
|---|---|---|
| Zeta | Sample | Material: Protein
Dispersant: Buffer (HEPES) General options: Smoluchowski Cell: Disposable folded capillary cell |
| Trend | Start Temperature: 20°C
End Temperature: 70°C Return to start temperature: Yes Temperature interval: 1.0°C | |
| Zeta potential measurement | Equilibration time: 300 seconds
Optimize measurements settings: Yes Number of measurements: 2 Append measurement number to sample name: Yes Measurement duration: 10-50 runs Automatic Voltage: No 40V Data processing: Monomodal |
| Type | Parameter | Setting |
|---|---|---|
| Size | Measurement Type | Temperature Trend - Zeta Measurement |
| Sample | Material: Protein
Dispersant: Buffer (HEPES) Cell: ZEN0040 capped | |
| Trend | Start Temperature: 20°C
End Temperature: 70°C Return to start temperature: Yes Check for aggregation: Yes Temperature interval: 1.0°C | |
| Size Measurements | Equilibration time: 300 seconds
Optimize measurements settings: Yes Number of measurements: 3 Append measurement number to sample name: Yes Allow results to be saved with correlation data only: Yes Measurement duration: Automatic Data Processing: Protein analysis |