In this application note, we discuss how Nanoparticle Tracking Analysis (NTA) is used to measure the size and concentration of nanoparticles used for drug delivery, such as liposomes, in order to determine efficacy and rate of uptake, degradation and clearance from the body.
The use of nanoparticles in drug delivery continues to grow rapidly. Nanoparticles offer excellent pharmacokinetic properties, controlled and sustained release, and targeting of specific cells, tissues or organs. Interest in nanoparticle drug delivery is also driven by the diminishing rate of discovery of new biologically active compounds that can be exploited therapeutically to treat disease. With fewer new drugs entering the market every year, interest in the use of nanoparticles’ versatile and multifunctional structures for the delivery of drugs is swiftly increasing. All these features can improve the efficacy of existing drugs (Malam et al., 2011).
Nanoparticles used in drug delivery have been defined as colloidal systems of submicron size that can be constructed from a large variety of materials in a large variety of compositions. Commonly defined nanoparticle vectors include: liposomes, micelles, dendrimers, solid lipid nanoparticles, metallic nanoparticles, semiconductor nanoparticles and polymeric nanoparticles. In their many guises, nanoparticles have been extensively employed to deliver drugs, genes, vaccines and diagnostics into specific cells/tissues. (Ram et al., 2011).
When considering a nanomaterial drug delivery system, size of the nanoparticle is a key parameter as it directly influences the processes of delivery, uptake, degradation and clearance from the body. For example, nanoparticles in the range of 30 nm to a few hundred nm in diameter can passively accumulate at the site of tumours due to leaky vasculature, phagocytosis favours particles >500 nm, whilst biliary and renal clearance occurs with particles <30 nm and <8 nm respectively. In addition, the liver has a lower uptake of smaller particles (25 nm and 50 nm) compared to larger particles (200 nm and 300 nm). Accurate measurement of the particles being administered is therefore imperative to many systems and processes.
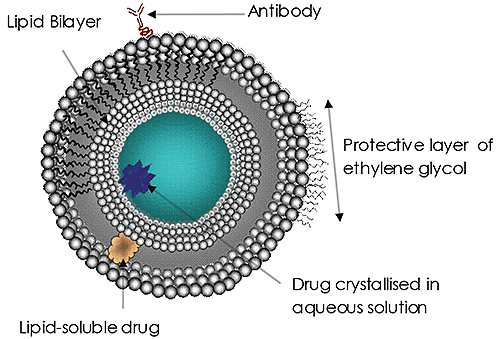
Liposomes (Figure 1) have been the subject of significant research and development efforts for many years and are currently the most common targeted drug delivery system. Liposomes have been approved as a delivery system for amphotericin B for fungal or protozoal infections, doxorubicin for breast cancer treatment, and for vaccines for hepatitis A and influenza. The use and potential of liposomes in drug delivery continues to grow in importance. The reasons are clear:
The size of the liposomes used is increasingly being recognized as an important factor in treatment efficacy. A drug delivery liposome’s size may affect its circulation and residence time in the blood, the efficacy of the targeting, its rate of cell absorption (or endocytosis) and, ultimately, the successful release of its payload. Such size considerations are hugely important to all nanoscale drug delivery systems.
|
Malvern’s NanoSight instrument range accurately and rapidly sizes and measures concentration of liposomes in water and other solvents. Only small volumes and very little sample preparation is required. The instruments enable individual liposomes in suspension to be visualized and their Brownian motion tracked – enabling particle size distributions, based on individual particles, to be built up in a matter of seconds.
|
In addition to size and concentration, NTA also provides the following parameters, simultaneously and particle-by-particle:
Poly(β-amino ester)s (PBAEs) are potential delivery systems for genetic therapies to treat various cancers. They have an advantage over some other systems in that many combinations of polymer with DNA can be made through the combinatorial route. They also have rapid release properties due to hydrolytic degradation, but this creates challenges for dosing, production and storage. Lyophilization is a typical storage method and NTA has been utilized to evaluate the effect of lyophilisation on aggregation (increase in size) and destruction (decrease in size) of PBAE-DNA nanoparticles (Tzeng et al. 2011 and Sunshine et al. 2012).
Poly(lactic-co-glycolic acid) (PLGA) is an FDA-approved drug delivery system. It breaks down into lactic acid and glycolic acid, both of which are endpoints of metabolic pathways in the body. PLGA has been used as a drug delivery system for amoxicillin and also for gonodotropin-releasing hormone for the treatment of advanced prostate cancer. The immunosuppressant mycophenolic acid has been encapsulated in PLGA with a view to decrease dosing levels and thus reduce toxic side effects. NTA was used to determine the size of these nanoparticles, a critical parameter to ensure good delivery and allow researchers to compare results across studies (Shirali et al. 2011).
The successful transport of molecules across the cell membrane is a key point in their delivery. In many cases, molecules alone cannot penetrate the cell membrane, therefore an efficient carrier is needed. Sokolova et al. (2012) have investigated calcium phosphate nanoparticles (diameter: 100 nm - 250 nm, depending on the functionalization) as versatile carriers for small and large molecules across cell membranes using a number of techniques including NTA, Dynamic Light Scattering (DLS) and Electron Microscopy (EM).
Ohlsson et al. (2012) reported on solute transport in the sub-100 millisecond timescale across the lipid bilayer membrane of individual proteoliposomes using NTA to check liposome stability and integrity.
In research on nanoparticles as gene delivery vehicles, Ghonaim and his co-workers have reported extensively on the use of NTA in their work on the effect of modifications to the chemistry of lipopolyamines and spermines in various non-viral plasmid DNA and siRNA delivery systems (Ghonaim et al., 2007a; Ghonaim et al., 2007b; Ghonaim et al., 2007c; Ghonaim, 2008; Ghonaim et al., 2009; Soltan et al., 2009; Ghonaim et al., 2010). Similarly, Ofek et al (2010) have employed NTA for the characterization of dendritic nanocarriers for siRNA delivery while Bhise measured particle size and size distribution by NTA in their study of gene delivery polymers in cell culture (Bhise et al., 2010). Bhise further extended this work to develop an assay for quantifying the number of plasmids encapsulated by polymer nanoparticles, in which he used NTA to determine the number density of plasmids per 100 nm nanoparticle (Bhise et al., 2011)
Wei et al. (2012), in exploring the challenges and opportunities in the advancement of nanomedicines, identified the need for robust methods for the accurate characterization of nanoparticle size, shape and composition, as well as particle engineering to maintain low levels of nonspecific cytotoxicity and to increase stability during storage.
Other examples of the importance of sizing and enumerating nanoparticulate drug delivery systems by NTA have been reported (Hsu et al., 2010; Park et al., 2010; Tagalakis et al., 2010).
1. Bhise NS, Gray RS, Sunshine JC, Htet S, Ewald AJ and Green JJ (2010) The relationship between terminal functionalization and molecular weight of a gene delivery polymer and transfection efficacy in mammary epithelial 2-D cultures and 3-D organotypic cultures, Biomaterials, doi:10.1016/j.biomaterials.2010.07.023
2. Bhise NS, Shmueli RB, Gonzalez J and Green JJ (2011), A Novel Assay for Quantifying the Number of Plasmids Encapsulated by Polymer Nanoparticles. Small. doi: 10.1002/smll.201101718
3. Ghonaim HM, (2008) Design and Development of Pharmaceutical Dosage Forms for Gene and siRNA Delivery, PhD Thesis University of Bath, Department of Pharmacy and Pharmacology, September 2008
4. Ghonaim HM, Li S and Blagbrough IS (2010) N1,N12-Diacyl Spermines: SAR Studies on Non-viral Lipopolyamine Vectors for Plasmid DNA and siRNA Formulation Pharmaceutical Research, Vol 27, (1) p17-29
5. Ghonaim HM, Li S, Soltan MK, Pourzand C and Blagbrough IS (2007a), Chain Length Modulation in Symmetrical Lipopolyamines and the effect on Nanoparticle Formulations for Gene Delivery, in British Pharmaceutical Conference BPC2007, Manchester, 10th Sept.
6. Ghonaim HM, Li S, Pourzand C and Blagbrough IS (2007b), Efficient Novel Unsymmetrical Lipopolyamine Formulations for Gene Delivery, in British Pharmaceutical Conference BPC2007, Manchester, 10th Sept.
7. Ghonaim H M, Li S, Pourzand C and Blagbrough IS (2007c), Formulation and Delivery of Fluorescent siRNA by Lipospermine Nanoparticle Complex Formation, in British Pharmaceutical Conference BPC2007, Manchester, 10th Sept.
8. Ghonaim H, Li S and Blagbrough IS (2009) Very Long Chain N4 , N9 -Diacyl Spermines: Non-Viral Lipopolyamine Vectors for Efficient Plasmid DNA and siRNA Delivery Pharmaceutical Research, Volume 26, Number 1, p19-31
9. Hsu J, Serrano D, Bhowmick T, Kumar K, Shen Y, Kuo Y C, Garnacho C and Muro S (2010) Enhanced Endothelial Delivery and Biochemical Effects of α-Galactosidase by ICAM-1-Targeted Nanocarriers for Fabry Disease Journal of Controlled Release, Article in Press, doi:10.1016/j.jconrel.2010.10.031
10. Malam Y, Lim E and Seifalian A (2011) Current trnds in application of nanoparticles in drug delivery, Current Medicinal Chemistry, Volume 18, Number 7, March 2011, pp.1067-1078 (12)
11. Ofek P, Fischer W, Calderon M, Haag R and Satchi-Fainaro R (2010) In vivo delivery of small interfering RNA to tumors and their vasculature by novel dendritic nanocarriers. FASEB J..doi 10:1096/fj.09-14964
12. Ohlsson G, Tabaei S, Beech JP, Kvassman J, Johansson U, Kjellbom P , Tegenfeldt JO and Höök F(2012) Solute transport on the sub 100 ms scale across the lipid bilayer membrane of individual proteoliposomes, Lab Chip, 2012, Accepted Manuscript, DOI: 10.1039/C2LC40518K
13. Park J, Gao W, Whiston R, Strom T, Metcalfe S and Fahmy TM (2010) Modulation of CD4+ T Lymphocyte Lineage Outcomes with Targeted, Nanoparticle-Mediated Cytokine Delivery, Mol. Pharmaceutics, 2011, 8 (1), pp 143-152
14. Ram M, Yaduvanshi KS, Yadav H, Singh N, Mangla G, Shivakumar H (2011) Nanoparticles, Promising Carriers in Drug Targeting: A review current drug therapy, Volume 6, Number 2, May 2011, pp.87-96(10)
15. Reshetov et al, Photochem Photobiol. 2012 Sep-Oct;88(5):1256-64. doi: 10.1111/j.1751-1097.2012.01176.x
16. Shirali, et al. Am J Transplantation. 2011; 11: 2582-2592
17. Sokolova V, Rotan O, Klesing J, Nalbant P, Buer J, Knuschke T, Westendorf AM and Epple M (2012) Calcium phosphate nanoparticles as versatile carrier for small and large molecules across cell membranes, Journal of Nanoparticle Research, Volume 14, Number 6 (2012), 910, DOI: 10.1007/s11051-012-0910-9
18. Soltan MK, Ghonaim HM, El Sadek M, Kull MA, El-aziz LA and Blagbrough IS (2009) Design and Synthesis of N4, N9-Disubstituted Spermines for Non-viral siRNA Delivery - Structure-Activity Relationship Studies of siFection Efficiency Versus Toxicity, Pharmaceutical Research, Volume 26, Number 2, p 286-295
19. Sunshine et al. PLoS ONE, 2012; 7(5):e37543. doi:10.1371 /journal. Pone .0037543
20. Tagalakis A D, Grosse S M, Meng Q-H, Mustapa M F M, Kwok A, Salehi S E, Tabor A B, Hailes H C and Hart S L (2010) Integrin-targeted nanocomplexes for tumour specific delivery and therapy by systemic administration, Biomaterials, Vol 32, Issue 5, February 2011, p1370-6
21. Tzeng et al. Biomaterials. 2011 August; 32(23): 5402-5410. doi:10.1016/j.biomaterials.2011.04.016
22. Wei A, Mehtala JG and Patri AK (2012) Challenges and opportunities in the advancement of nanomedicines, Journal of Controlled Release, http://dx.doi.org /10.1016/j.jconrel.2012.10.007