Dynamic Light Scattering (DLS) is a mature and proven technology for nanomaterial characterization. Nanoparticle Tracking Analysis (NTA) is a more recent addition to the spectrum of particle characterization techniques and has many similarities to DLS, leading to some confusion as to their relative capabilities. The broad range of measurement capabilities for each technique is complementary in nature, providing a wealth of valuable information. The differences between the two techniques allow the data from one technology to validate and support the other.
Dynamic Light Scattering (DLS) is a mature and proven technology for nanomaterial characterization. Nanoparticle Tracking Analysis (NTA) is a more recent addition to the spectrum of particle characterization techniques and has many similarities to DLS, leading to some confusion as to their relative capabilities. The broad range of measurement capabilities for each technique is complementary in nature, providing a wealth of valuable information. The differences between the two techniques allow the data from one technology to validate and support the other.
The Zetasizer Nano Dynamic Light Scattering instrument family and the NanoSight Nanoparticle Tracking Analysis instrument range complement each other well, providing a suite of measurement capabilities useful to many researchers interested in characterizing nanoparticle and bioparticulate systems. This whitepaper will explore how the unique capabilities of these two product ranges, when used in conjunction, can provide a more comprehensive understanding than either technique alone.
Both techniques are similar because they both rely on the Brownian motion of and light scattering from the particle. Both also use the Stokes-Einstein equation to relate diffusion to size (hydrodynamic diameter). In practice though, they are quite different techniques, since NTA produces number-based distribution and DLS produces an intensity-based distribution. NTA provides a particle-by-particle measurement whilst DLS provides an ensemble measurement.
Analysis of polystyrene latex standards, commonly used to validate the operation of all particle sizing instruments, shows how well the techniques agree on measurement of the primary particle size of the sample. In the below example, using 200 nm polystyrene latex beads, the distribution width is slightly different according to each of the techniques. NTA reports a narrower distribution, which more closely matches the (number-based mean) certified value from the manufacturer. The broader distribution for a monodisperse material like this is expected for DLS, and the manufacturer's certificate usually includes an additional set of specifications for DLS measurements.
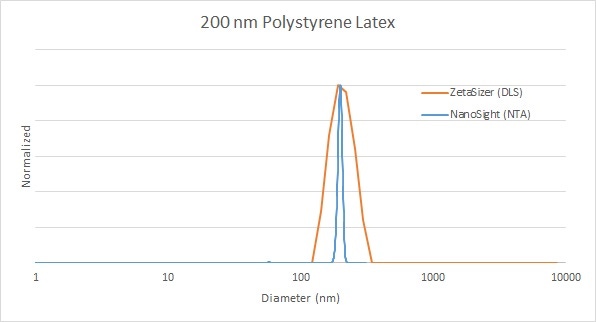
Figure 1: Comparison of 200 nm polystyrene latex standard size distribution analysis by DLS (red) and NTA (blue)
Extracellular vesicles (exosomes or microvesicles) are biological vesicles that are usually between 50 nm and 200 nm in diameter. NTA provides a measurement of the total concentration, a critical parameter in extracellular vesicle research, as well as a high resolution size distribution of the vesicle population. In this example, DLS showed a broad peak representing the exosomes, as well as a second peak around 30 nm that was invisible to NTA. These smaller particles are not exosomes, but might be of interest in particular applications. Only DLS has the necessary sensitivity to measure these smaller nanoparticles and to report them in a mixture with the primary particles of interest. In this situation, DLS can be used to elucidate the total sample composition, but NTA is then used to further resolve the peak of main interest, as well as provide the concentration result.
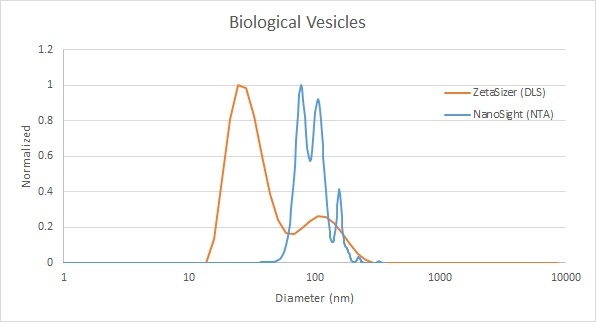
Figure 2: Comparison of biological vesicle measurement results by DLS (red) and NTA (blue)
In the below example, 50 nm gold nanoparticles were functionalized with antibodies. NTA was able to fully characterize the size distribution, including the small aggregate tail, as well as provide a total concentration. DLS detected the primary peak, but was also sensitive to a very small number of larger particles. As DLS is an intensity-based measurement and the scattering intensity increases to the sixth power of the diameter, the approximately tenfold difference in size leads to a very bright particle that is very easily detected by DLS, even at very low concentrations. DLS is a sensitive technique for monitoring aggregate presence.
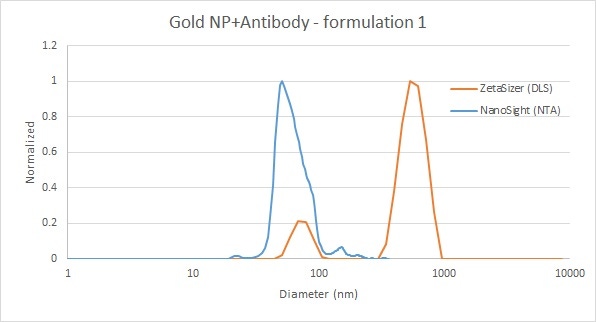
Figure 3: Comparison of gold nanoparticle and antibody mixture measurements by DLS (red) and NTA (blue)
The second example (below) shows analysis of a similar material, but DLS has also detected a peak at around 10 nm, which is most likely free antibody that did not bind the gold particles. In this case, the smaller peak is below the lower detection limit for the NTA technique, but can be clearly and reliably detected by DLS.
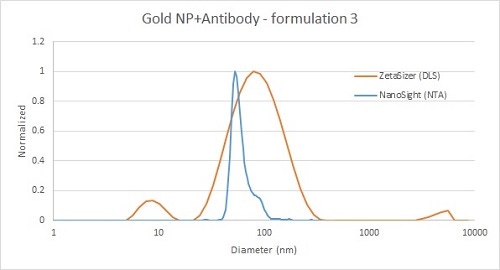
Figure 4: Comparison of gold nanoparticle and antibody mixture measurement by DLS (red) and NTA (blue) - with free antibody detected by DLS only
The following series of examples shows the analysis by DLS and NTA of different formulations with differing size distributions. In the first example, NTA provides high-resolution measurement of the primary peak, whereas DLS offers a broader distribution intensity - weighted towards larger particles, as well as the presence of a very low concentration of large particles (Figure 5).
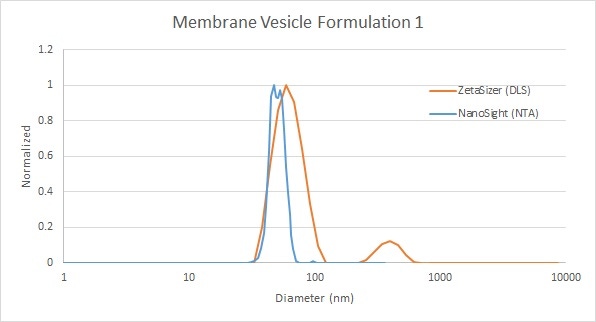
Figure 5: Comparison of membrane vesicle analysis by DLS (red) and NTA (blue)
In the second example, DLS is able to pick up both the micron-scale aggregates and the free lipid below 10 nm, whereas NTA has the resolution to give a highly-resolved measurement of the primary peak (Figure 6), which represents the vesicles themselves. So in this case, NTA provides more information about the vesicles, whereas DLS provides additional information about a wider range of constituents in the sample.
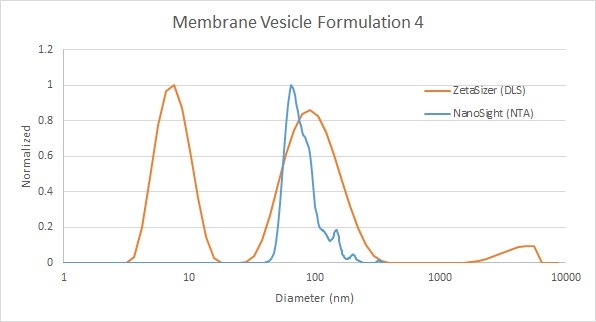
Figure 6: Comparison of membrane vesicle analysis by DLS (red) and NTA (blue) - with free lipid picked up by DLS
Example 3 (Figure 7) shows a similar sample with both very small and very large particles in the mixture, as well as a bimodal distribution detected by DLS. NTA measurement of the main peak provides a high resolution size distribution with multiple modes and a quantitative measure of the large-size tail within that size segment. This high resolution provides added value for more precise information about this vesicle peak.
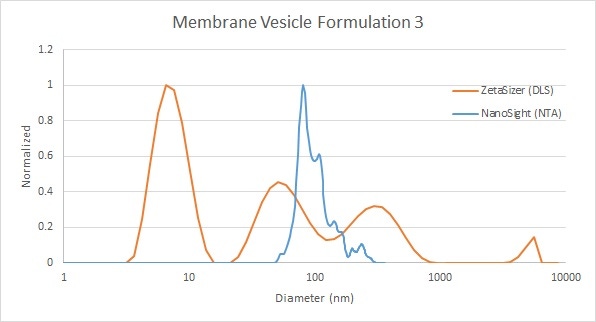
Figure 7: Comparison of membrane vesicle analysis by DLS (red) and NTA (blue)
This study is extracted from a paper which focuses on the functionalization of gold nanoparticles using an antibody (mouse IgG)[1]. The data show the change in absolute size at different antibody concentrations. Both techniques mirror exactly the same trend, with DLS showing amplified changes likely due to the intensity-weighted emphasis towards larger sizes in a sample, as seen in previous examples.
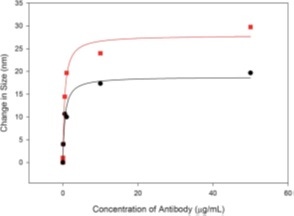
Figure 8: Size change as a function of antibody concentration, as measured by DLS (red) and NTA (black).
This study evaluated hybrid nanoparticles as a platform for intranasal vaccination with subunit antigens[2]. Size and zeta potential were studied as a function of hyaluronic acid (HA) polymer concentration. As an increasing amount of HA was added to the unilamellar liposomes, their size gradually increased. Addition of more than 300 µg of HA caused non-homogenous aggregation shown by an abrupt and significant increase in particle size and polydispersity index (PDI) values. This is further explained by the zeta potential measurements, which show consistently positive values until addition of more than 300 µg of HA. Above this concentration, the zeta potential decreases sharply, eventually reaching negative values at high loadings. During this transition, it is most likely that the system will aggregate significantly, due to the reduced electrostatic stabilization forces. The NTA results generally confirmed these DLS (and electrophoretic light scattering for zeta potential measurements) readings and are a useful orthogonal technique for confirmation. The NTA number distributions were able to show the increasing aggregate tail as the HA was added.
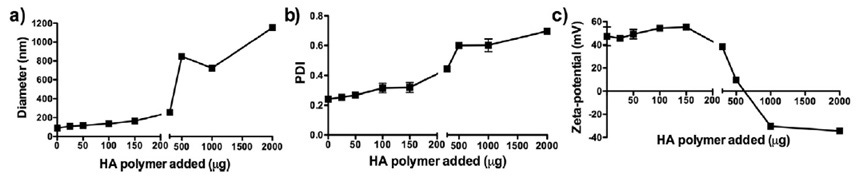
Figure 9: DLS characterization of liposomes with varying amounts of HA polymer: a) z-average size; b) PDI; c) zeta potential
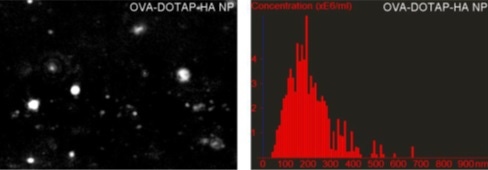
Figure 10: NTA characterization of liposomes with varying amounts of HA polymer confirmed DLS results
The examples discussed here show some of the challenges that can be resolved with a broader range of characterization information and a combination of technologies. The below list may be useful in establishing the strengths of each individual technique only one technology is available.
Consider NTA when:
you need number-based high-resolution sizing
you need accurate distribution shape or width, or where the tails of the distribution are important
you need particle concentration or count
you need a direct view of sample when results from other techniques are ambiguous
your sample is fluorescent
your sample is too dilute for DLS
you want to confirm or validate DLS measurements
Consider DLS when:
you need excellent reproducibility
you need accurate and repeatable mean size and polydispersity index measurements according to ISO standards
comparison of different batches or quality control is of interest
you need a noninvasive, rapid and reliable assessment of particle size
size versus time and size versus temperature is of interest for stability assessment
a broad concentration range is desired or the sample cannot be diluted for NTA
you need to cover the broadest size range, especially below 30 nm and above 800 nm
1. James A. & Driskell J. Monitoring gold nanoparticle conjugation and analysis of biomolecular binding with nanoparticle tracking analysis (NTA) and dynamic light scattering (DLS), DOI: 10.1039/c2an36467k
2. Fan Y. et al. Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens, Journal of Controlled Release 208 (2015) 121–129