The stability of colloids in a formulation is important for the quality of a product. Processes such as aggregation can lead to final products which are not optimum for use, such as paint, with visible lumps, or inks which do not re-suspend fully and therefore could potentially block inkjet nozzles.
With more complex products, multiple materials are combined from various sources, and the conditions under which they are transported and stored in can vary. Consequently, when combining these component parts, the pH and ionic strength of the formulation can vary. To avoid catastrophic product failure, such as the scenarios mentioned above, it is important to gain an understanding of the components of the system and how they behave across varying pH and ionic strengths to ensure that they remain stable across any chemical changes they may be subjected to during production.
This application note discusses the use of the multipurpose titrator MPT-3 in studying the effect of salt concentration on stable pH ranges. The MPT-3 is an accessory available for the Zetasizer Advance product range.
The stability of colloids in a formulation is important for the quality of a product. Processes such as aggregation can lead to final products which are not optimum for use, such as paint, with visible lumps, or inks that do not re-suspend fully and therefore could potentially block inkjet nozzles.
With more complex products, multiple materials are combined from various sources, and the conditions under which they are transported and stored in can vary. Consequently, when combining these component parts, the pH and ionic strength of the formulation can vary. To avoid catastrophic product failure, such as the scenarios mentioned above, it is important to gain an understanding of the components of the system and how they behave across varying pH and ionic strengths to ensure that they remain stable across any chemical changes they may be subjected to during production.
This application note discusses the use of the multipurpose titrator MPT-3 in studying the effect of salt concentration on stable pH ranges. The MPT-3 is an accessory available for the Zetasizer Advance product range.
Solutions of NaCl were made up at concentrations of 0.010 M, 0.050 M, and 0.100 M respectively, and anatase TiO2 was added at 0.1 % w/v concentration. These suspensions were dispersed using an ultrasonic bath, and 10 mL aliquots were titrated from pH 10 to 4 using titrants of 0.250 M and 0.025 M HCl with a pH step size of 0.5 at 20°C. Three repeat zeta potential measurements were made at each pH point in the titration with a delay of 60 seconds between each repeat measurement. All titrations were conducted using folded capillary cells (DTS1070) on a Zetasizer Ultra-Red instrument with a multipurpose titrator MPT-3 and an auto degasser.
The results from the pH titrations using the different NaCl concentrations are shown in Figure 1, and the isoelectric points (the point of zero zeta potential) are presented in Table 1.
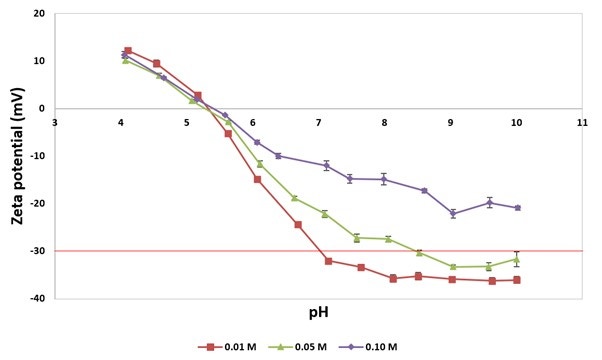
Figure 1: pH titrations of 0.1 % w/v anatase in 0.01 M (red; square), 0.05 M (green; triangle), and 0.10 M (purple; diamond) NaCl solutions, from pH 10 to 4, with pH step sizes of 0.5 pH units, and three repeats measured at each point. Error bars show standard error, and the red line at -30 mV represents the boundary between an aqueous dispersion being stable or unstable [1-3].
| NaCl Concentration (M) | Isoelectric Point (pH) | Stable pH Range (pH) |
|---|---|---|
| 0.01 | 5.31 | ≈ 7 to 10 |
| 0.5 | 5.28 | ≈ 8.5 to 10 |
| 0.1 | 5.40 | None found |
| Table 1: Isoelectric points and sable pH ranges for the pH titrations of 0.1 % w/v anatase in solutions of varying NaCl concentrations. | ||
As can be seen in Figure 1, all samples start will a negative zeta potential at pH 10, experience a pH range where the zeta potential does not vary, with the values then increasing until they pass through the isoelectric point at around pH 5.3. As the salt concentration increases, the initial plateau period reduces, and the initial zeta potential values increase to give three clearly different trends.
This observed change in the measured zeta potential is a direct consequence of the increasing salt concentration in the samples. The measured zeta potential describes the net charge of the particles in the system it is being measured in. By increasing the NaCl content, counter ion concentration increases resulting in lower measured zeta potentials. The H+ ions, introduced by the acidic titrants, will affect the zeta potential of the sample in a similar way. The ions present in the system are interacting with the TiO2 in a non-specific way as the isoelectric points are not affected [1].
The consequence of this is that the pH range of stability will be reduced with increasing salt concentration and could even lead to complete instability of the product, as shown in Figure 1 and Table 1. Zeta potential values of greater than 30 mV or lower than -30 mV are considered as stable [2-4] and, as can clearly be seen by the red line at -30 mV in Figure 1, the 0.01 M and 0.05 M samples transition this stability value, whilst the 0.10 M sample yielded zeta potential values >-30 mV throughout the titration. This demonstrates the influence of the composition of a buffer and the importance of the zeta potential value on the stability of a sample.
The results obtained in this study show that the ionic strength has a significant effect on the stability of a colloid in a formulation, and can restrict the stable pH range when increased, or even make the sample completely unstable. Therefore, it is important to be aware of the ionic strength and pH ranges which different components in a formulation will be exposed to, in order to maintain a stable formulation.
[1] Measurement and Interpretation of Electrokinetic Phenomena (2005) Pure Appl. Chem. 77, 1753-1805.
[2] B. Derjaguin, L. Landau (1941) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes, Acta Physico Chemica URSS 14, 633.
[3] E.J.W. Verwey and J. Th. G. Overbeek (1948) Theory of the stability of lyophobic colloids, Elsevier, Amsterdam.
[4] Zeta Potential of Colloids in Water and Waste Water (1985), ASTM Standard D 4187-82, American Society for Testing and Materials.