Dr Olivier Deschaume, Dr Kirill Shafran, Professor Carole Perry, School of Biomedical and Natural Sciences, Nottingham Trent University, Clifton Lane, Clifton, Nottingham, UK (This application note is an excerpt taken from O. Deschaume, K.L. Shafran and C.C. Perry (2006) Langmuir 22, 10078-10088).
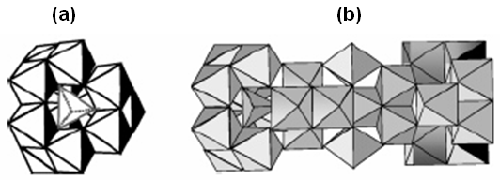
In mildly acidic conditions at concentrations above the solubility limit of aluminum hydroxide, monomeric Al species, such as the hexaaquocation[Al(H2O)6]3+ and its hydrolysis products ([Al(H2O)5(OH)]2+, [Al(H2O)4-(OH)2]+, can undergo a succession of condensation reactions. These reactions can lead to the formation of small oligomeric Al species, such as Al dimers and trimers [1] and further transformation into large Keggin ions Al13-mers [2] and the recently characterized Al30-mer (figure 1). These have been employed in a number of applications including antiperspirant actives [3], preparation of Al2O3 nanoparticles [4] and composite materials [5].
|
Previous studies on protein-aluminum interactions have largely concentrated on elucidating bioavailability of this element and absorption/elimination pathways in living organisms [6]. Fewer studies have investigated interactions of Al species with proteins and other biopolymers with regard to materials chemistry applications. A significant effect of the surface charge of aluminum hydroxide on predominantly electrostatic interactions with BSA has been previously demonstrated [7,8]. The substantial impact of aluminum hydroxide as the adjuvant on antigen structure and function has also been reported [9].
A better understanding of the interactions of aluminum species with biomolecules is a necessary pre-requisite for successful application of a combined biomimetic-nanotectonic approach to advanced Al-containing materials. This application note describes the effect of a model protein, bovine serum albumin (BSA), on the generation and properties of hybrid Al-protein composite materials formed from various high-purity Al-containing aqueous nanosized precursors.
In this study, size characterization of the aluminum nanoparticles and Al-BSA samples was made using the technique of dynamic light scattering (DLS). Zeta potential measurements of the Al-BSA samples were made using the technique of laser Doppler electrophoresis. Further information on these techniques is contained in the technical notes "Dynamic Light Scattering: An Introduction in 30 Minutes" and "Zeta Potential: An Introduction in 30 Minutes" available from the Malvern Instruments Ltd website.
Details of the preparation of the Al13- and Al30-mers can be found in the literature [10]. The final Al concentration in all samples was 0.4M.
A series of Al-BSA solutions were prepared at room temperature by the addition of different amounts of fresh BSA stock solution to the appropriate model Al-containing system. All Al-BSA solutions were vigorously stirred during and after mixing of Al-containing and BSA solutions for 60 seconds using a vortex mixer and left aging at room temperature (25 ± 0.2 °C) for 24 hours. The final aluminum concentration in the Al-BSA solutions was 0.2M and the BSA concentration was varied from 0 to 25mg/mL.
Dynamic light scattering measurements of the Al13-, Al30-mers and Al-BSA complexes were made on a Zetasizer Nano ZS at a temperature of 25°C. This instrument contains a 4mW He-Ne laser operating at a wavelength of 633nm and an avalanche photodiode (APD) detector. The scattered light was detected at an angle of 173° and this novel optics arrangement maximizes the detection of scattered light while maintaining signal quality. This provides the exceptional sensitivity that is required for measuring the size of nanoparticles, such as the Al clusters measured in this study.
Zeta potential measurements of the Al-BSA complexes were made using the Zetasizer Nano ZS with disposable capillary cells at a temperature of 25°C. The measured electrophoretic mobilities were converted into zeta potential values using the Smoluchowski approximation [11].
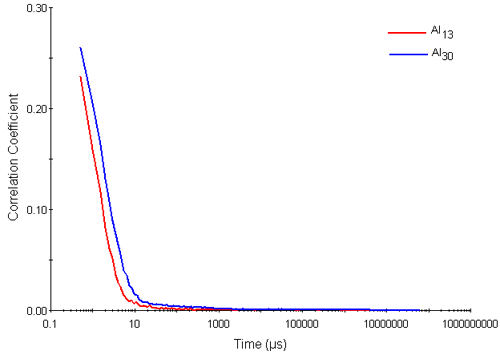
Figure 2 shows typical correlation functions obtained for the Al13 and Al30-mers. They exhibit two visible decay rates. The rapid decay rates seen in delay times up to 10 microseconds are interpreted as arising from the diffusion of the Al nanocluster particles. The slower decay rates seen near to the baseline of the correlation functions (at correlator delay times of between 10 and 1000 microseconds respectively) are interpreted as aggregates.
|
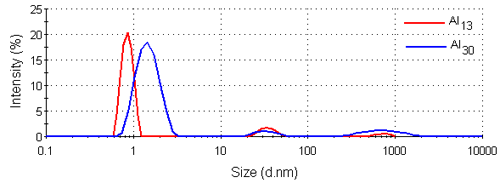
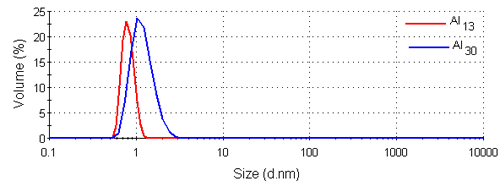
Figure 3 shows the intensity size distributions for the Al13 and Al30-mers obtained from the analysis of the correlation functions with a non-negative least squares fit [12, 13]. Both samples have main peaks around 1nm in diameter. These intensity size distributions were converted into volume using Mie theory [14]. The volume size distributions obtained are shown in figure 4 and consist of single peaks. The intensity and volume peak means and modes are summarized in table 1.
|
|
DLS measurements of the Al-BSA complexes are shown in Figure 5. Once BSA was added to the Al nanoparticles, the average particle size increased to ≈10.7 ± 0.5nm. For the Al13-mer-BSA samples, the mean size of the suspension did not change significantly with further increase of BSA concentration. However, in the case of Al30-containing samples, the average particle size increased at BSA concentrations above 17.5mg/ml. The mean diameter of BSA was measured to be ≈ 8 to 9nm. These larger than expected mean particle sizes could arise from the adsorption of Al13-mer clusters on the surface of BSA molecules that are negatively charged under mildly acidic conditions (pH < 5.0). These clusters would be expected to have a larger size than the BSA molecule.
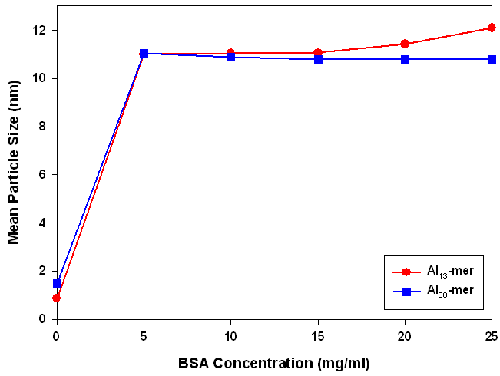
|
| Sample | Intensity | Volume | ||
|---|---|---|---|---|
| Al13-mer | 0.86 | 0.85 | 0.81 | 0.77 |
| Al30-mer | 1.49 | 1.44 | 1.20 | 1.03 |
This hypothesis is supported by zeta potential measurements of the Al-BSA complexes (figure 6).The zeta potential values for the Al polyoxocation-BSA complexes were positive at all BSA concentrations measured, with only small differences between the Al13- and Al30-containing samples being observed. The zeta potential of solutions of the Al13 and Al30 polyoxocations without BSA could not be measured as the size of these particles was too small to be detected.
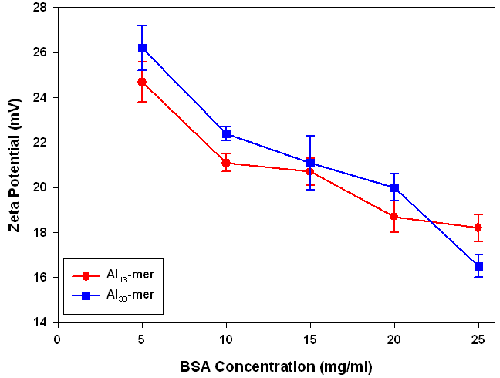
|
Measurements of the zeta potential of BSA solutions of similar concentration gave negative zeta potential values of -8.6 ± 0.4 mV. The positive zeta potentials values obtained for the Al-BSA complexes can therefore be explained by the adsorption of Al polyoxocations on the surface of the protein. With increasing concentration of BSA, the ratio of Al polyoxocations to protein falls resulting in a decrease in the zeta potential.
These zeta potential measurements provide supporting evidence for the predominantly electrostatic interactions between the Al containing species and BSA. Correlation of increasing BSA concentration with decreasing zeta potential indicates that the negative charge of BSA is being cancelled by the Al polyoxocations.
The Zetasizer Nano has been successfully used to determine the size of Al13 and Al30-mers and characterize the size of complexes formed between these aluminum polyoxocations and bovine serum albumin. The non-invasive backscatter (NIBS) optics of the Zetasizer Nano provides the exceptional sensitivity required to characterize particles around 1 nanometer in size such as the aluminum polyoxocations used in this study.
The measurement of the zeta potentials of Al-BSA mixtures has given an insight into the mechanisms of interaction involved in the formation of polyoxocation-protein complexes.
[1] D.F. Baes Jr and R.E. Mesmer in The Hydrolysis of Cations (1996), Wiley-Interscience, New York.
[2] J.W. Akitt (1989) Prog. Nucl. Magn. Reson. Spectrosc. 21, 1-149.
[3] J.J. Fitzgerald in Antiperspirants and Deodorants (1988), Marcel Dekker, New York.
[4] N. Yao, G. Xiong, Y. Zhang, M. He, and W. Yang (2001) Catal. Today 68, 97-109.
[5] J.-H. Son, H. Choi, Y.U. Kwon and O.H. Han (2003) J. Non-Cryst. Solids 318, 186-192.
[6] G. Berthon (2002) Coord. Chem. Rev. 228, 319-341.
[7] A. Wittayanukulluk, D. Jiang, F.E. Regnier and S.L. Hem (2004) Vaccine 22, 1172-1176.
[8] R.H. Al-Shakhshir, F.E. Regnier, J.L. White and S.L. Hem (1995) Vaccine 13, 41-44.
[9] L.S. Jones, L.J. Peek, J. Power, A. Markham, B. Yazzie and C.R. Middaugh (2005) J. Biol. Chem. 280, 13406-13414.
[10] O. Deschaume, K.L. Shafran and C.C. Perry (2006) Langmuir 22, 10078-10088.
[11] IUPAC Technical Report: Measurement and Interpretation of Electrokinetic Phenomena (2005) Pure Appl. Chem. 77, 1753-1805.
[12] Charles L. Lawson and Richard J. Hanson (1995) Solving Least Squares Problems, SIAM Society for Industrial & Applied Mathematics.
[13] S. Twomey (1997) Introduction to the mathematics of inversion of remote sensing and indirect measurements, Dover Publications.
[14] Mie G (1908) Ann. Physik, 4: 377-445.