Colloidal gold is a dispersion of suspended nanoparticles of gold which exhibit interesting properties [1]. The color of the sample is determined by the size and shape of the gold particles present [2]. Figure 1 shows colloidal gold suspensions of various particle sizes. Sizes less than 5 nm are yellowish in color, 5 nm to 20 nm tend to be reddish in color whereas particles greater than 100 nm are bluish in appearance.

|
Gold particles in aqueous media attain a negative charge which gives them a strong affinity for various biological macromolecules such as proteins and antibodies [3]. For this reason, colloidal gold is currently used in a variety of biotechnological applications such as DNA-conjugates, imaging probes and diagnostic agents [1,4,5]. In addition, colloidal gold suspensions are being developed for use in advanced electronics and coating applications [6].
Size characterization of colloidal gold is of great importance in order to ensure that the particles are homogenous in diameter and that there are no aggregates present in the dispersion. Size characterization is popularly done using electron microscopy techniques [1,2]. Figure 2 shows a transmission electron micrograph of a colloidal gold sample. Even though the individual gold particles are clearly visible, the majority of them exist as clumps consisting of 2 or more particles.

|
Even though electron microscopy is an excellent technique for the visualization of particles, it is poor from a statistical point of view as only tens or hundreds of particles are measured. The technique will give a number-based particle size as the numbers of different sized particles are counted.
Dynamic light scattering (DLS) is a non-invasive technique for measuring the size of nanoparticles in a dispersion. The technique measures the time-dependent fluctuations in the intensity of scattered light from a suspension of particles undergoing random, Brownian motion. Analysis of these intensity fluctuations allows for the determination of the diffusion coefficients, which in turn yield the particle size through the Stokes- Einstein equation.
This application note discusses the size characterization of colloidal gold using dynamic light scattering and highlights the differences in the results that might be obtained compared with electron microscopy techniques.
All measurements reported in this application note were performed on a Zetasizer Nano S at 25°C. The Nano S contains a 4 mW He-Ne laser operating at a wavelength of 633 nm and an avalanche photodiode (APD) detector. The scattered light was detected at an angle of 173°.
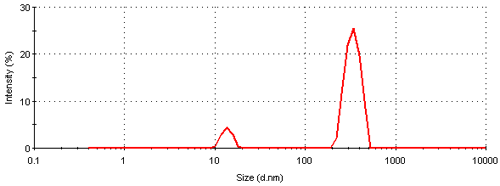
Figure 3 shows the intensity particle size distribution obtained for a colloidal gold sample measured on a Nano S. The plot shows the relative percentage of light scattered by particles (on the Y-axis ) in various size classes (on the X-axis). The size distribution obtained is a bimodal with peak means of 13.6 and 339 nm respectively. A summary of the peak analysis of the distribution can be found in table 1.
|
| Peak 1 | Peak 2 | |||
|---|---|---|---|---|
| Mean (nm) | % | Mean (nm) | % | |
| Intensity | 13.6 | 11 | 339 | 89 |
| Volume | 13.0 | 91 | 363 | 9 |
| Number | 12.4 | 100 | - | - |
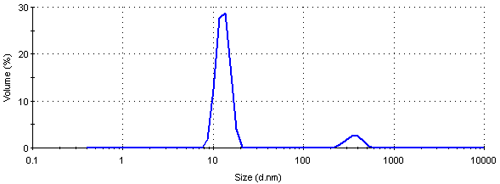
The intensity size distribution obtained implies that there are significant aggregates present in this sample. However, conversion into a volume (or mass or weight) based size distribution (figure 4) shows that the aggregates are present in low concentration. The transformation from the intensity data into volume is performed using Mie theory and this conversion requires the particle refractive index (n) and absorption (k) values. The optical properties used for the conversion from intensity to volume in this study were 0.2 (n) and 3.32 (k) respectively [9]. The volume size distribution obtained shows that, on a mass basis, over 90% of the sample consists of small particles around 13 nm.
|
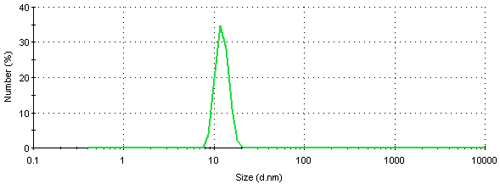
The result can be further converted into a number based size distribution which is shown in figure 5. This distribution is monomodal with a peak mean at 12.4 nm. The result suggests that if this sample was characterized using a number based technique, such as electron microscopy, the vast majority of particles visible would be small ones. The presence of large particles would only be seen if sufficient numbers were counted. Even though, on a number basis, this sample contains very few aggregates, they scatter a significant amount of light contributing to the major peak in the intensity size distribution (figure 3). Therefore such a sample would be expected to give quite different results when analyzed by dynamic light scattering and electron microscopy.
|
On a further note, if the sample illustrated in figure 2 was to be measured by dynamic light scattering, it would not be possible to resolve different sized peaks for the various particle species i.e. single particles, aggregates of 2 particles, aggregates of 3 particles etc. Dynamic light scattering is a low resolution technique being able to resolve materials with a factor of 3 difference in their sizes.
So a mixture of single particles and aggregates made of 2, 3 or 4 particles would be expected to give a broad single peak with the result obtained being influenced by the larger particles present as they would scatter the majority of the light. The z-average diameter and polydispersity index values are sensitive to the presence of aggregates. The z-average diameter is the mean hydrodynamic diameter and the polydispersity index is an estimate of the width of the distribution. Both of these parameters are calculated according to the International Standard on dynamic light scattering, ISO22412 [10].
The technique of dynamic light scattering is ideally suited to the size determination of colloidal gold. It is very sensitive to the presence of aggregates of particles and the z-average diameter and polydispersity index values could be used as a way of determining sample homogeneity.
For monodisperse and monomodal samples, the results obtained from dynamic light scattering and electron microscopy should be very similar. However, for samples which are polydisperse, the results obtained from dynamic light scattering will be larger than those obtained from electron microscopy studies due to the contribution of scattering from the larger particles
[1] M.A. Hayat (1989) Colloidal Gold: Principles, Methods and Applications, Academic Press, New York.
[2] K. Miura and B. Tamamushi (1953) J. Electron Microscopy 1, 36-39.
[3] M. Horisberger and M.F. Clerc (1985) Histochem and Cell Biol. 82, 219-223.
[4] A. Csaki, R. Möller and W. Fritzsche (2002) Expert Rev. Mol. Diagn. 2, 89-94.
[5] R. Tanaka, T. Yuhi, N. Nagatani, T. Endo, K. Kerman, Y. Takamura and E. Tamiya (2006) Anal. Bioanal. Chem 385, 1414-1420.
[6] T. Sato and H. Ahmed (1997) Applied Phys. Letters 70, 2759-2761.
[7] A.N. Shipway, E. Katz and I. Willner (2000) 1, 18-52.
[8] P. Mulvaney, M. Giersig and A. Henglein (1992) J. Phys. Chem. 96, 10419- 10424.
[9] L. G. Shulz (1954) J. Opt. Soc. Am. 44, 357-362 and 362-368.
[10] International Standard 22412:2008 Particle size analysis - Dynamic light scattering (DLS)