Differential scanning calorimetry (DSC) is an established biophysical assay technique for the characterization of protein stability . A protein's stability is a critical quality attribute (CQA) which is characterized throughout biopharmaceutical drug discovery and development, to help decide if it should be chosen to advance in the pipeline, and also to help guide selection of the process and formulation conditions needed to maintain its stability. In a DSC system, the protein in solution is placed in the DSC cell and heated at a constant rate, resulting in the protein unfolding due to thermal denaturation. The protein’s heat capacity (Cp) changes as the protein denatures. The peak of a DSC thermogram represents the protein's thermal transition midpoint (TM). For a protein which reversibly denatures, TM is the temperature where 50% of the protein is in its native (folded) conformation, and 50% is in its denatured (unfolded) conformation. TM is considered a good indication of thermal stability – the higher the TM, the more thermally stable the protein. Even if a protein is irreversibly denatured during a DSC experiment, the TM still provides important insights into its thermal stability. Multi-domain proteins (like antibodies) typically have more than one peak on a DSC thermogram, so more than one TM can be determined.
The first automated DSC system for biopharmaceutical applications, MicroCal VP-Capillary DSC, was launched in 2002. With automation, DSC quickly performs TM screening for biopharmaceutical preformulation and formulation development. Data from MicroCal VP-Capillary DSC systems are used to evaluate protein drug products for: candidate selection/protein engineering; process development; higher order structure (HOS) characterization during comparability studies and manufacturing support; HOS characterization for biosimilar development.
In 2017, Malvern Panalytical launched the MicroCal PEAQ-DSC Automated system. Compared to the MicroCal VP-Capillary DSC, the MicroCal PEAQ-DSC Automated system has new features and improvements designed to improve productivity during biopharmaceutical discovery and development, generating high-quality, reproducible, and reliable DSC data which can be used for regulatory submissions. MicroCal PEAQ-DSC Automated systems are essential additions to the 'biophysical toolbox' in biopharma, CROs and CDMOs, and core facilities at academic and government research laboratories.
Differential scanning calorimetry (DSC) is an established biophysical assay technique for protein stability characterization during biopharmaceutical drug development. DSC data are used to advance the most stable and 'developable' proteins into the pipeline, and optimize the process and formulation conditions to maintain protein stability during manufacturing and storage.
DSC determines the thermal transition midpoint (TM). For a protein which reversibly denatures, TM is the temperature at which 50% of the protein is in its native (folded) conformation, and 50% is in its denatured (unfolded) conformation. TM is considered a good indication of thermal stability – the higher the TM, the more thermally stable the protein. Even if a protein is irreversibly denatured during DSC, the TM provides important insights into its thermal stability. Multi-domain proteins, like antibodies, typically have more than one unfolding domain, and DSC detects multiple TMs. In a recent survey of biopharmaceutical scientists, DSC was rated as a 'very useful' to 'extremely useful' biophysical tool for candidate selection, formulation development, product characterization, comparability, and biosimilarity[1].
The MicroCal VP-Capillary DSC system, launched in 2002, was the first automated DSC for biopharmaceutical applications. MicroCal VP-Capillary DSC systems perform TM screening during biopharmaceutical preformulation and formulation development. Data from MicroCal VP-Capillary DSC systems are also used to evaluate protein stability during candidate selection, protein engineering, process development, higher order structure (HOS) characterization during comparability studies, manufacturing support, and biosimilar development. MicroCal VP-Capillary DSC systems have been installed in biopharmaceutical facilities, CROs and CRDOs worldwide, and their use is cited in hundreds of journal articles, book chapters, and reviews.
In 2017, Malvern Panalytical launched the MicroCal PEAQ-DSC Automated system. Compared to the MicroCal VP-Capillary DSC, the MicroCal PEAQ-DSC Automated system has new features designed to improve productivity during biopharmaceutical discovery and development, generating high-quality, reproducible, and reliable DSC data.
1. DSC data analysis in the regulated environment: PEAQ-Compliance
DSC data are increasingly included in regulatory submission packages for new biopharmaceutical drugs and biosimilars. DSC stability data are also used in documentation for proposed manufacturing changes to established processes, to demonstrate that the changes have no effect on the final drug product. There is also interest in including DSC as a Quality Control assay during biopharmaceutical manufacturing.
In the regulated environment, the instrument user needs to demonstrate data integrity. The US FDA provides guidelines in their document “Data integrity and Compliance with cGMP: Guidance to Industry (April 2016)”[2]. One requirement is that all record creation and modification is controlled and auditable. This requirement is covered in FDA 21 CFR Part 11 or EU GMP Annex 11, which were written to enable pharmaceutical companies to submit electronic data. MicroCal VP-Capillary DSC systems use Origin 7 software, which does not support 21 CFR Part 11 compliance requirements.
PEAQ-Compliance software, which is available as an optional feature for MicroCal PEAQ-DSC systems, has tools which support FDA 21 CFR Part 11 compliance:
Another component of 21 CFR Part 11 compliance is Performance Qualification (PQ) of the instrument, which includes regular performance checks. MicroCal PEAQ-DSC systems include PEAQ-Performance, a new tool for conducting and evaluating performance check data (see #2).
Performance checks: Typical standard operating procedures (SOPs) for MicroCal VP-Capillary DSC systems include regular 'performance check' experiments, using a well-characterized 'standard' with an established DSC thermogram. These experiments serve as a check of the DSC instrument, which is important to generate high-quality, reliable, and reproducible DSC data. Regular performance checks enable user confidence in DSC data, and reduce the need for multiple replicate experiments. Performance checks are also a component of Performance Qualification (PQ), required for data analysis in a regulated environment (see #1 above). MicroCal VP-Capillary DSC users were not automatically notified of failed performance checks during the course of DSC experiments, and experiments had to be repeated after the instrument issues were addressed.
PEAQ-Performance is a new tool provided with MicroCal PEAQ-DSC Automated systems. Performance check experiments on standards are incorporated into the sample set at desired intervals. PEAQ-Performance software automatically assesses the standard DSC data. If the performance check fails (does not meet defined tolerances of DSC parameters), the instrument operator has the option to program the instrument to either clean the DSC cells, or stop further experiments, or continue the rest of the programmed experiments, or be alerted via email if the performance check fails. For the last of these options, the operator can decide the next steps after notification.
DSC cell cleaning: Clean DSC cells are critical for generating high-quality DSC data. A typical SOP with MicroCal VP-Capillary DSC automated systems includes rinsing the DSC cells with Contrad 70 (or Decon 90) solution after scanning a sample, and performing one or two buffer-buffer reference scans before the next sample scan. The buffer-buffer scans serve as an additional instrument performance check to make sure the DSC cells are clean and the instrument is functioning. For rigorous cleaning of the MicroCal VP-Capillary DSC cells, users add detergent solution in the 96-well plate, and program a cleaning 'scan', followed by at least one buffer-buffer scan. These SOPs take time to perform, use space in the sample trays, and productivity and sample throughput are reduced.
PEAQ-Performance includes three pre-programmed DSC cell cleaning protocols, optimized for the MicroCal PEAQ-DSC Automated system: 1) water rinse; 2) detergent wash (followed by water rinse); 3) detergent scan (fill cells with detergent, perform scan, rinse with water). The new autosampler with MicroCal PEAQ-DSC systems has improved sample handling, resulting in faster, effective cell cleaning, reducing carry-over and contamination. Using an optimized DSC cell cleaning protocol makes it possible to increase productivity by performing three (or more) DSC experiments with high quality data, without buffer-buffer scans in between. Figure 1 shows thermograms for 16 DSC scans of eight different batches of ribonuclease A, with no buffer-buffer scans between the protein scans.
DSC system warm-up: MicroCal VP-Capillary systems require multiple 'warm-up' scans to ensure optimal performance. MicroCal PEAQ-DSC systems include an warm-up feature which detects when the instrument is ready and will automatically start experiments. This reduces the warm-up scan time and provides confidence in data quality.
Fast scan rates: For TM screening experiments, MicroCal PEAQ-DSC systems generate high-quality thermograms using fast scan rates (up to 240 °C/h). Using fast scan rates is another way to increase productivity and save time.
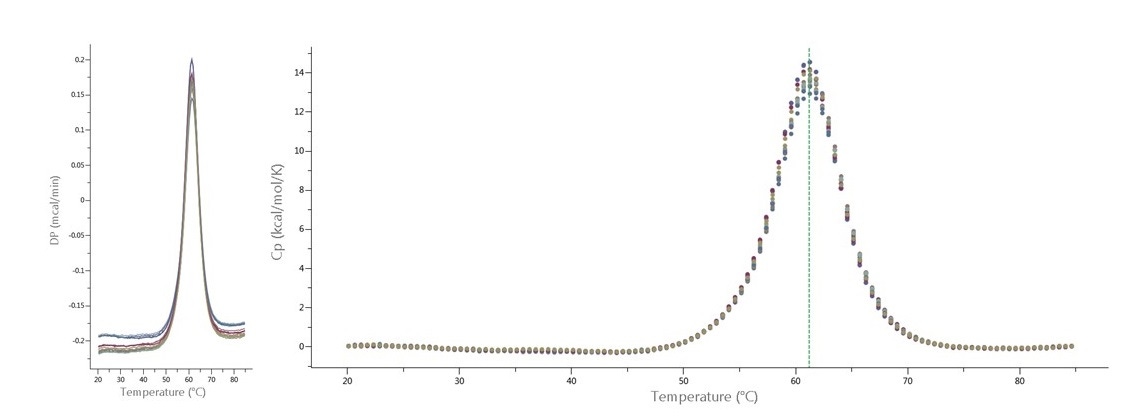
Figure 1. Sixteen thermograms of ribonuclease A (60 µM, or 0.88 mg/mL) analyzed with MicroCal PEAQ-DSC Automated system. DSC temperature range was 15-85 °C, at 200 °C/h scan rate. DSC cells were cleaned with 'detergent wash' after each sample. Buffer-buffer scans were performed before first protein scan and after final protein scan. Left: Raw data as displayed by PEAQ-Smart software. Data are two replicate scans of 8 different batches of protein. Right: after data analysis and fitting with PEAQ-Smart software. Green line represents TM determined by PEAQ-Smart.
Figure 1 shows sixteen thermograms of ribonuclease A performed on a MicroCal PEAQ-DSC Automated system at 200 °C/h scan rate. The experiments with MicroCal VP-Capillary DSC automated system at 200 °C/h scan rate would take about 33 hours to complete a total of 40 DSC scans, including four warm-up scans, sixteen buffer-buffer scans (before each sample scan), two detergent scans, and one performance check scan (with matched buffer-buffer scan).
Using the MicroCal PEAQ-DSC Automated system, the sixteen sample thermograms required 20 hours to complete a total of 24 scans (including two warm-up scans, two buffer-buffer scans, two detergent scans, and performance check protein scan). In this example there is a time saving of approximately 13 hours using the MicroCal PEAQ-DSC Automated system, which allows an additional 15 scans to be completed per day, a productivity increase of around 75%.
MicroCal PEAQ-DSC Automated systems include PEAQ-Smart software for programming DSC experiments. The software has sample templates, allowing rapid re-use of common configuration elements. The template setup allows easy moving and duplicating of experiments. Buffer-buffer, sample, and performance check standard experiments are color coded. If desired, each DSC experiment can be unique in scan rate, temperature range, cell cleaning method, and other parameters. The methods can be designed on the user’s computer and transferred to the instrument, and also saved for future use.
For studies involving reversibility of unfolding, the user is able to program the MicroCal PEAQ-DSC Automated system for multiple rescans of the same sample, using different temperature ranges for the rescans – note that MicroCal VP-Capillary DSC Automated system control software can only program rescans with the same settings as the initial scan.
Users can also program the MicroCal PEAQ-DSC Automated system to perform downscans (cooling scans) after an upscan (heating) - this option was not available in VP-Capillary DSC Automated system.
Analysis of multiple data files from MicroCal VP-Capillary DSC can be a bottleneck, and frequently takes multiple mouse-clicks to process the data. PEAQ-Smart data analysis software quickly processes multiple data sets simultaneously, including subtraction of buffer-buffer baseline, creation of integration baselines, and fitting data to determine TM and overall enthalpy. PEAQ-Smart also includes a report designer to present the data.
Monoclonal antibodies typically exhibit two to four unfolding transitions in DSC, and each transition has its own TM. MicroCal VP-Capillary DSC data analysis software includes a 'hit box' to identify TMs from DSC thermograms. The hit box can 'miss' transitions with shallower peaks in the thermogram, requiring the user to manually process each thermogram. MicroCal PEAQ-DSC systems include the new PEAQ-Finder tool - this new algorithm automatically determines more than one TM from DSC thermograms, including peaks that Origin software would miss. Figure 2 shows a comparison of TM finding for PEAQ-Finder and the hit box for Origin.
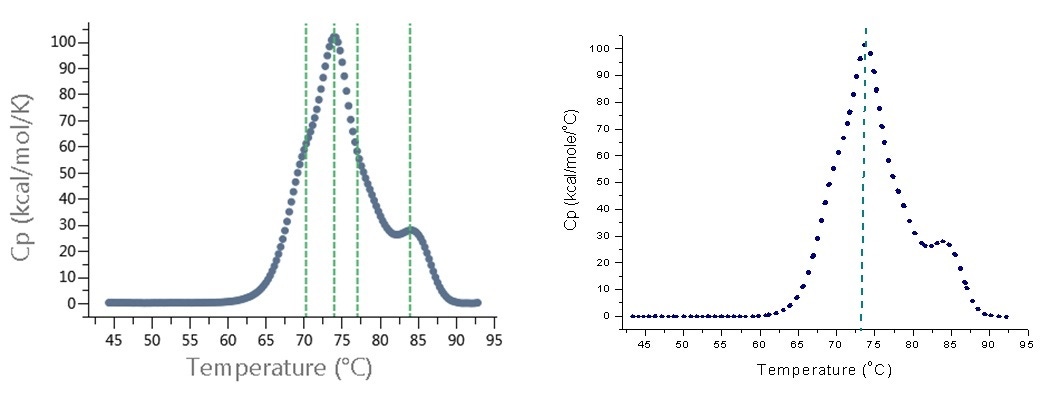
Figure 2. TM identification in DSC thermogram of monoclonal antibody sample. Left: After analysis with PEAQ-Smart and PEAQ-Finder software tool from MicroCal PEAQ-DSC system. The software identified four TMs, indicated by green dashed lines. Right: After analysis with Origin DSC data analysis and hit box from MicroCal VP-Capillary DSC. The software identified only one TM, indicated by green dashed line.
DSC is frequently incorporated in biophysical and HOS assays for biopharmaceutical comparability, to demonstrate that the manufactured protein product is 'highly similar' when compared to a reference lot of the same protein. When substantial changes are made to the manufacturing process, a comparability exercise is conducted to evaluate the impact of the change(s) on the critical quality attributes of the protein product.
A biosimilar is a biopharmaceutical that it is highly similar to a previously approved biological product usually made by another company (the parental, innovator, or reference biopharmaceutical). DSC is commonly used as a HOS biophysical assay to show that a biosimilar has a highly similar DSC profile 'fingerprint' when compared to the parental drug.
MicroCal PEAQ-DSC systems include PEAQ-Compare, a new software tool which performs data analysis and evaluation on a set of DSC thermograms, and provides an objective, quantitative similarity comparison to a reference DSC thermogram. PEAQ-Compare is useful for comparability and biosimilarity studies.
The new autosampler with the MicroCal PEAQ-DSC Automated system allows the user to place 325 µL of protein sample in the tray well (per scan), compared to the 370 -400 µL needed for MicroCal VP-Capillary DSC Automated systems.
Typical SOPs for the MicroCal PEAQ-DSC system require protein concentrations of 0.5 to 1 mg/mL for high-quality DSC data. For many protein samples, it is possible to achieve high-quality DSC thermograms with 0.05 mg/mL protein (see Figure 3), and for TM screening, even lower protein concentrations can be used.
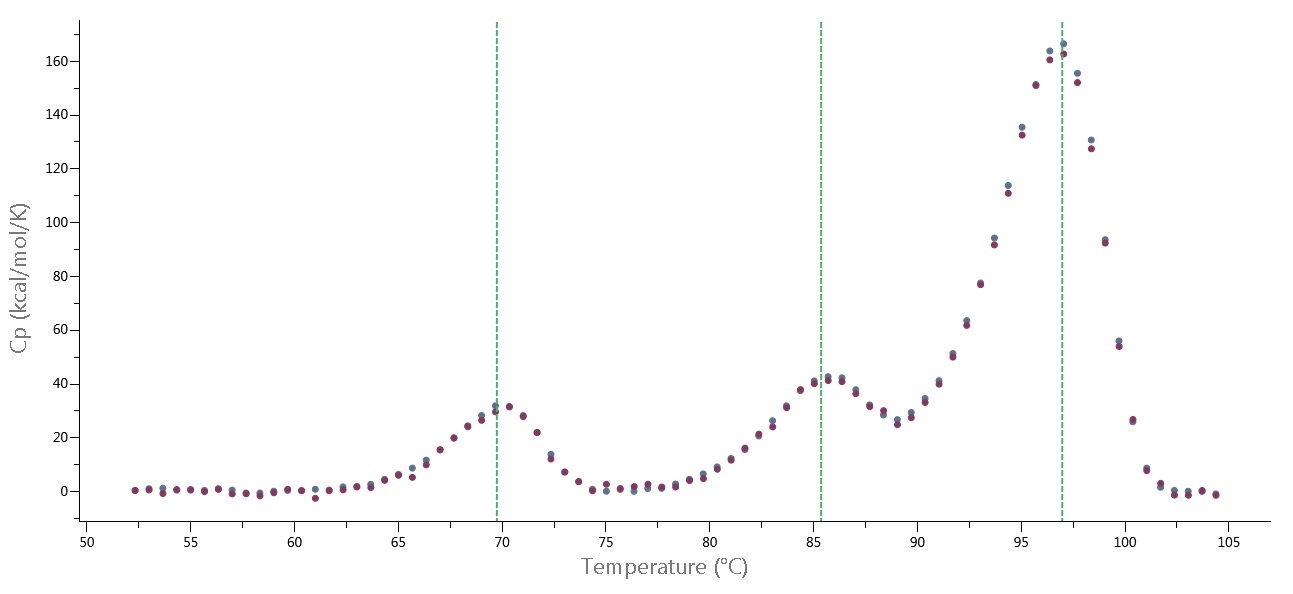
Figure 3. Data from MicroCal PEAQ-DSC Automated system, at 240 °C/hour scan rate. Thermograms of NISTmAb at 0.167 mg/mL (red) and 0.0555 mg/mL (blue) after data analysis with PEAQ-Smart software. Green lines represent TMs determined by PEAQ-Smart.
If DSC is frequently used at your facility, and is critical to your workflow, any instrument down-time will cost time and money, and slow down your institute’s drug development or research projects.
MicroCal PEAQ-DSC Automated systems have a new, more robust autosampler design, a new controller, and more robust firmware. The controller includes a computer that can network to your institution’s servers, and the installed software is email-ready. The autosampler has several components (including the needle seal) which are more robust and have longer lifetimes, compared to MicroCal VP-Capillary DSC automation.
The new MicroCal PEAQ-DSC Automated system has a number of features for increasing productivity, discussed above. Software licensing allows data analysis offline, even under 21 CFR Part 11 compliance, and provided software allows data to be saved directly to your corporate network.
NOTE: Malvern Panalytical also provides the MicroCal PEAQ-DSC system without an autosampler. For more information on the non-automated MicroCal PEAQ-DSC system, see product information at malvernpanalytical.com or contact your regional Malvern Panalytical sales office.
Specification/feature | MicroCal PEAQ-DSC Automated | MicroCal VP-Capillary DSC (with autosampler) |
Tools to support 21 CFR Part 11 compliance | PEAQ-Compliance (optional feature) | Not available |
Performance check: Programmed standard experiments autoanalyzed | Included in PEAQ-Performance. If performance check fails, user has option to program cell cleaning, stop further DSC scans, continue experiments, or notify user by email | Not available |
Multiple standardized cell cleaning methods | Included in PEAQ-Performance. Three pre-programmed cell cleaning options | Two cell cleaning options |
Automated warm-up | Included in PEAQ-Performance | Not available |
Data analysis | PEAQ-Smart: Fast, intuitive, automated, data analysis with PEAQ-DSC software | Origin software. Limited functionality |
TM determination for DSC transitions | PEAQ-Smart and PEAQ-Finder, automated TM determination of multiple thermograms | Origin software hit box |
Automated subtraction of matched buffer-buffer scan | Included in PEAQ-Smart. More options for choosing buffer-buffer scan for subtraction compared to Origin software | Included in Origin software, limited options to choose buffer-buffer scan |
Automated integration baseline generation and determination of ∆H | Included in PEAQ-Smart, improved algorithms and to select pre- and post-transitions; progress, spline, and linear integration baselines | Included in Origin software, limited options compared to PEAQ-Smart; one integration baseline option |
Email sent to user | Available | Not available |
Networking software | Yes: PEAQ-DSC software is ready to network | Limited with Origin software |
Control software for easy, intuitive experimental setup | Included in PEAQ-Smart. Includes templates. More options and features in experimental design compared to VP-Viewer | Included in VP Viewer control software, limited options in experimental design |
Video-enabled workflows | Included with PEAQ-Smart | Not available |
Compare DSC thermograms to reference sample DSC scans | Included with PEAQ-Compare | Not available |
Data presention | Included with PEAQ-Smart, more options | Limited options |
Report designer | Included with PEAQ-Smart | Not available |
Sorting/'binning' results after data analysis | Included with PEAQ-Smart | Limited options with Origin software |
Recommended sample volume in tray wells | 325 μL | 370-400 μL |
Fully integrated automated liquid handling system | NEW AUTOSAMPLER. Improvements include: Injection needle with bottom-detector (permits reduced sample volume in well plates); faster wash station optimized DSC cell cleaning; new needle seal (longer lifetime, reduced carry-over) | Complete precision XYZ robotic arm. Three thermostatically controlled drawers, each holding two 96-well microtiter plates. 10-port injection valve. Integrated wash station for rapid, high volume cleaning. Injection syringe. |
Controller | Windows 10 OS. All PEAQ-DSC software pre-loaded. | Windows 7 OS. All Origin software pre-loaded. |
Downscanning for reversibility studies | PEAQ-Smart includes option to schedule downscanning after upscanning experiment | Not available |
Program unique scan conditions in set of experiments | Included in PEAQ-Smart. More options and features in experimental design compared to VP-Viewer | Included in VP Viewer control software, limited options in experimental design |
Rescan samples for reversibility studies | Included in PEAQ-Smart. Rescans can be different scan conditions compared to 1st scan | Included in VP- Viewer. Rescans have to be identical scan conditions compared to 1st scan |
Pressure perturbation calorimetry (PPC) | Not available for MicroCal PEAQ-DSC Automated systems (Available as an option for non-automated MicroCal PEAQ-DSC systems) | Not available |
DSC cell composition | Tantalum-61 |
DSC cell design | Fixed-capillary cell design |
DSC cell active cell volume | 130 µL |
Models available for DSC data analysis | Includes: two-state unfolding; non-two-state unfolding; KD of ligand binding by TM shift. |
Operating temperature range for DSC scans | - 10 to + 130 oC (Using upscan mode at 60°C/h. When using upscan mode at 200°C/h, the upper temperature limit is 115°C) (NOTE: Operation between -10 to +2 °C on request upon instrument order) |
Minimum response time | 5 Sec * |
Noise | 0.05 μCal/°C * |
Baseline repeatability | 1.5 μCal/°C * |
Measurement repeatability | <0.2 µCal/°C *(rescans of stable buffer) |
Measurement reproducibility (intra-instrument) | <0.08 °C St. Dev. TM and < 2% RSD on ∆H (Ribonuclease A) |
System reproducibility (inter-instrument) | System reproducibility <0.1 °C St. Dev. TM and < 5% RSD on ∆H ( Ribonuclease A on MicroCal PEAQ-DSC alpha instruments) |
Multiple feedback modes | Three (passive, high gain, low gain) |
Maximum scan rate for upscanning | 240 oC per hour |
Power feedback compensation | Yes |
N2 or other inert gas supply for cell pressurization | Required (gas supply and regulator supplied by customer, more information on request) |
Typical sample concentration range | 0.01-10 mg/mL (sample dependent) |
Number of samples (capacity) | 6 x 96 well plate. Each scan requires 2 wells – total of 288 samples. |
Sample storage temperature range | 4°C - 40°C |
*More details available on product specification sheet, available from Malvern Panalytical
MicroCal PEAQ-DSC Automated systems include many new features that make DSC even easier to use, and produce reliable, reproducible DSC data. The systems also have tools for using DSC in a regulated environment. New tools such as PEAQ-Compliance, PEAQ-Smart, PEAQ-Finder, PEAQ-Performance and PEAQ-Compare, plus new hardware, make the new MicroCal PEAQ-DSC Automated system an essential addition to your biophysical toolbox in biopharmaceutical labs, CROs and CMOs, and core facilities at academic and government research labs.
Gabrielson, J.P., and Weiss, W.F.J. Pharm. Sci. 104, 1240-1245 (2015) doi: 10.1002/jps24393.
http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm495891.pdf