The Zetasizer µV is specifically designed to be compatible with a wide range of cuvette volumes, including a dedicated 2μl quartz cuvette. This cell is not disposable and must be thoroughly cleaned and dried between samples to avoid cross contamination. The 2μl sample volume is so small that great care must be taken to avoid sample or rinse fluid remaining in the cell, as the sample will become heavily contaminated or diluted.
The small volume also means that the sample solvent or dispersant will evaporate quickly when the stopper is removed, particularly if measurements have been conducted at elevated temperatures, following an aggregation point experiment for example. It is best to use the system to reduce the cell temperature to ambient at the end of the experiment, and before the stopper is removed. If the sample is allowed to dry onto the cuvette wall it will be much more difficult to clean effectively, if at all. This means that it is important to clean the cuvette as soon after the measurement as possible.
If the cell has not been used for an extended period or its cleanliness is unknown, it is recommended that the cell is cleaned thoroughly before use. Less rigorous cleaning procedures may be acceptable if the usage history is known to be up to a good standard.
|
To thoroughly clean the cell, a recommended cleaning agent is 2 % Hellmanex® III solution, made from the concentrate by diluting with demineralised water. Soaking for up to 15 minutes with cleaning solution at up to 60ºC increases its effectiveness. To ensure the 2μl sample well at the bottom of the cell is reached by the solution, it is useful to use a pipette with a 200μl tip or smaller, or a mini Pasteur pipette, to fill the sample well from the bottom up, as surface tension will prevent the well filling if the solution is simply poured in. Do not leave the cleaning solution in the cell so long that it evaporates, as the alkaline cleaner could damage the cell surface.
Ultrasonication is not recommended for this cell. The cuvette must be thoroughly rinsed to remove all of the cleaning solution. This should be done repeatedly with UHQ-water. This rinsing should be performed at least 5 times to fully rinse the cell. A pipette should repeatedly be used to make sure the solution in the sample well is being thoroughly exchanged. Looking through the window in the cell it should be possible to see the pipette tip in the sample well while rinsing the cell.
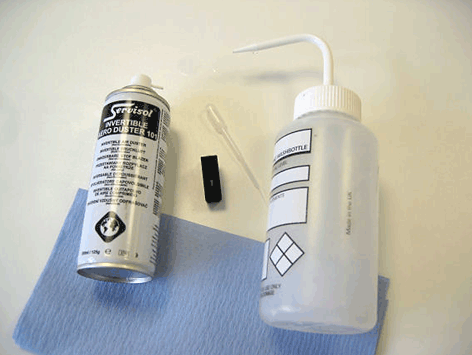
Once the cell has been cleaned and rinsed, all the solution in the cell must be removed in order to avoid any dilution by the remaining cleaning fluid. Initially this should be done by flicking the cell to remove the excess liquid. Using a pipette with a tip 200μl or smaller or a mini Pasteur pipette, any remaining liquid in the cell should be removed taking special care over the sample well itself. An air duster can also be used to remove the last of the rinsing fluids from the cell.

|
Finally, it is important to clean the outside of the cell itself in order to avoid dirt contributing to the scattering. This can be done by wiping with an optical tissue moistened with alcohol

|
Thorough cleaning of the 2 µl cell is necessary to remove the risk of cross-contamination of samples. The cell should also be carefully dried to reduce the risk of any sample dilution. Care taken in cleaning the cell will improve the quality of data measured and extend its lifetime.