Dynamic Light Scattering proves an excellent technique for making measurements of the thickness of an adsorbed Dextran layer used to enhance the stability of the casein stabilized emulsions used in food, cosmetics and pharmaceuticals.
Layer thickness varied from 2nm for unconjugated beta lactoglobulin up to 22nm for protein complexed with 440kDa dextrane. Asymptotic behavior indicated complete surface coverage.Surface layer increase is slightly larger than expected hydrodynamic diameter of the dextrane part, likely due to fnon-sphericity and surface orientation or dextran confirmation change on binding to beta lactoglobulin.
Dr Tim J. Wooster, Professor Mary A. Augustin, Food Science, Australia (CSIRO)
This application note summarizes measurement of the adsorbed layer thicknesses of β-lactoglobulin-dextran conjugates as a function of the change with dextran molecular weight. These measurements should yield information on the structure of this layer in terms of its thickness and orientation.
Caseins and whey proteins are widely used as emulsifiers in food, cosmetic and pharmaceutical industries. The efficiency of these emulsifiers is a function of their interfacial structure [1,2]. The caseins form a thick fluid interfacial layer which extends 13 to 15nm into the bulk aqueous phase [3]. Emulsions formed using casein are generally stable when the steric layer extends into the bulk. They become unstable upon the collapse of this steric layer as a result of lowering the pH or in the presence of ethanol [4].
The whey proteins form a thin (2 to 3nm) dense interface with little protein protruding into the bulk [4]. Whey protein emulsions are stabilized by electrostatic charge [5]. They flocculate upon removal of this charge when the pH approaches the isoelectric point or in the presence of electrolyte [3,5]. The stability of whey protein emulsions can be enhanced by attaching a steric layer through reaction with a polysaccharide via a carbodiimide linkage or the Maillard reaction [6]. Numerous studies report improved emulsion capacity and stability when β-lactoglobulin is chemically linked to carbohydrates [6-8]. Most studies examine emulsions with low protein contents (0.1 wt%) and the enhanced emulsion stability is measured using a turbimetric method [8, 9]. The enhanced emulsion stability observed using this method is more often related to particle size (emulsion capacity) rather than to changes in colloidal stability [6, 10, 11]. Therefore the question of "how bulky does the attached carbohydrate have to be in order to impart high steric stability to emulsions?" remains unanswered.
Good models for studying this phenomenon are protein-dextran conjugates, as the dextran is predominantly linear and readily available in defined molecular weights. There is little knowledge of the interfacial structure of protein-dextran conjugates with respect to its orientation and thickness as a function of molecular weight and its effect on emulsion stability. Dynamic light scattering (DLS) can be used to gain an understanding of the hydrodynamic thickness of the interface by examining emulsifiers adsorbed onto latex [12, 13]. DLS measures the time-dependent fluctuations in the intensity of scattered light from a suspension of particles undergoing random, Brownian motion. Analysis of these intensity fluctuations allows for the determination of the diffusion coefficients, which in turn yield the particle size through the Stokes- Einstein equation [14].
b-Lactoglobulin (Lot No. 025K7017), and dextrans D35, D65 and D500 were obtained from Sigma Aldrich. The dextrans had average molecular weights (Mw) of 36, 60 and 440KDa respectively. Dextrans D20 and D150 were obtained from Pharmacosmos and had average molecular weights of 18.3 and 153KDa respectively. Polystyrene latex spheres (anionic, 166 and 60nm diameter) were obtained from Duke Scientific. All buffers and salts were analytical grade and were also obtained from Sigma Aldrich.
Extensive details of the preparation, evaluation of the extent of conjugation and purification of β-lactoglobulin-dextran conjugates can be found in the literature [15].
Maillard conjugate adsorbed layer thickness was assessed by adsorbing the protein/purified conjugate onto monodisperse polystyrene latex beads and measuring the particle size using dynamic light scattering. Adsorbed layer thickness was estimated from the difference in the hydrodynamic diameter of the naked latex bead and latex beads with adsorbed layers. All DLS measurements were carried out on a Zetasizer Nano ZS using a scattering angle of 173°. Samples were measured after 5 minutes equilibration within the instrument at 25°C and the results are reported as the average of 3 measurements. The error between measurements on different sized lattices was ±0.3nm. All samples were prepared in a buffer of 20mM Tris/HCl and 30mM NaCl which were filtered using a 0.45μm filter prior to use.
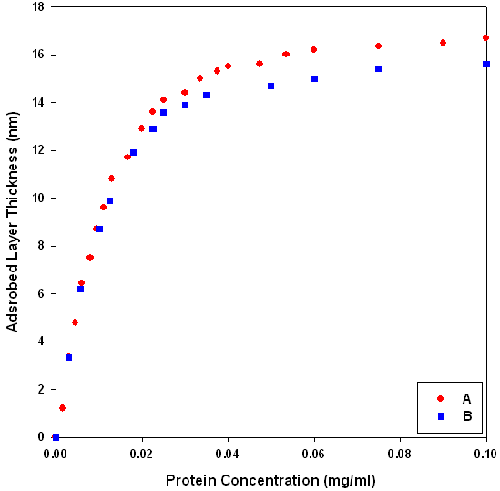
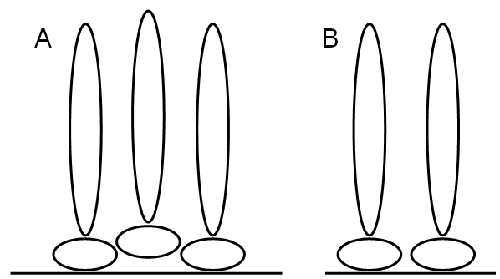
The hydrodynamic thickness of b-lactoglobulin-dextran Maillard conjugate interfaces was assessed using dynamic light scattering. The adsorbed layer thickness was estimated from the difference in size between latex spheres and those with protein adsorbed onto their surface. The final thickness of the conjugate layer was taken from the plateau region of protein concentration versus layer thickness curves. There is some difference in layer thickness depending on how the conjugate was applied (figure 1). Layers formed by sequential addition of conjugate to the one batch of latex had a higher plateau thickness than those prepared fresh at each concentration (using fresh latex and a single aliquot of solution). This may be because the orientation of sequentially adsorbed conjugates is affected by the nature of the initial adsorbed conjugate layer. This may result in uneven packing at the interface and hence an artificially higher layer thickness (figure 2). Therefore, all subsequent adsorption profiles were prepared fresh at each protein concentration.
|
|
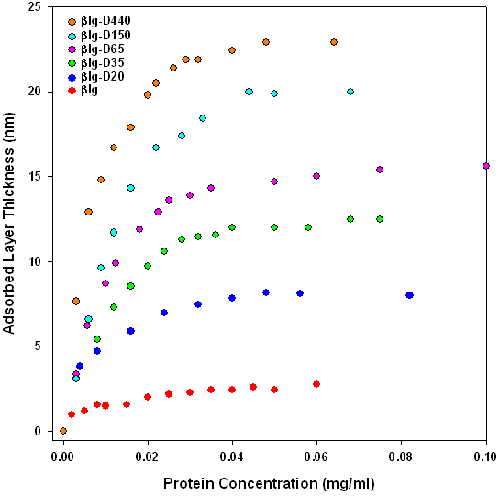
Figure 3 shows the adsorbed layer thickness versus protein concentration (not conjugate concentration) of β-lactoglobulin and of β-lactoglobulin-dextran Maillard conjugates (βlg-D20, βlg-D35, βlg-D65, βlg-D150 and βlg-D500). This figure serves to determine the concentration where a plateau in the layer thickness is obtained. The reproducibility of these plateau thicknesses between different preparations on the two different sized latex spheres (60 and 160nm), was found to be about 0.6nm. The adsorption of β-lactoglobulin results in the formation of a 2.9nm thick layer on the latex. This thickness measurement corresponds with that obtained by other authors [16]. The adsorption profiles shown in figure 3 indicate that conjugation of dextran to β-lactoglobulin increases layer thickness. Table 1 summarizes the measured and calculated hydrodynamic dimensions of β-lactoglobulin and dextran in solution and conjugates as adsorbed layers. These results show that the thickness of the layer is influenced by the dextran molecular weight Mw.
|
| Material Properties | β-Lactoglobulin-Dextran Conjugate Properties | |||
|---|---|---|---|---|
| Molecular Weight (kDa) | Hydrodynamic Diameter (nm)a | Adsorbed Layer Thickness (nm)b | Dextran Steric Layer Thickness (nm)c | |
| β-lactoglobulin | 18.3 | 5.6 (dimer) | 2.9 | 0 |
| D20 | 18.2 | 5.0 | 8.2 | 5.3 |
| D35 | 36.0 | 8.0 | 12 | 9.1 |
| D65 | 60.3 | 10 | 15.7 | 12.9 |
| D150 | 132 | 14.9 | 20 | 17 |
| D440 | 440 | 18 | 23 | 20.1 |
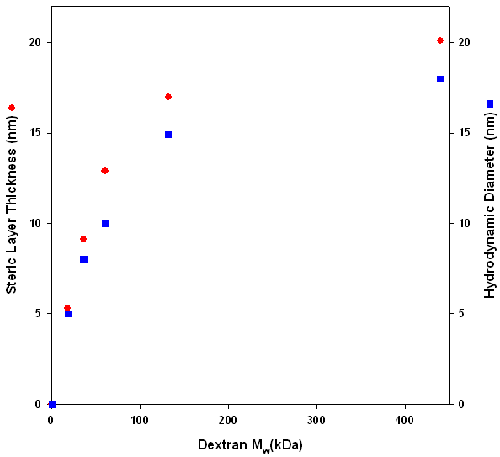
Figure 4 shows that there is a correlation between the increase in layer thickness and dextran solution hydrodynamic diameter. However, as the molecular weight of the dextran increases, there is a difference between the solution hydrodynamic diameter and the steric layer thickness. The difference could be because of artifact generated by the assumption that the particle is spherical. In solution, dextran behaves as an ellipsoid with one axis longer than the other two. However, DLS treats dextran as a sphere and averages all three axes, resulting in a smaller apparent hydrodynamic diameter. When dextran is conjugated to b-lactoglobulin, it is possible that the long axis of dextran is perpendicular to the latex surface. In this case, the size that is measured is the long axis and this gives rise to the difference in size between the two measurements. Alternatively, the difference could result from a change in dextran conformation caused by the conjugate adsorbing onto the latex. When the conjugate is adsorbed onto latex, the osmotic pressure of the microenvironment of the dextran is higher than that of dextran in the bulk. This might cause the dextran to elongate to increase solvent interaction.
|
Dynamic light scattering is a useful technique for studying the adsorption of molecules onto the surface of particles. The work summarized in this application note has measured the effect of dextran attachment to β-lactoglobulin as a function of dextran molecular weight on the interfacial thickness of these emulsifiers. The results show that the attachment of dextran to β-lactoglobulin increased steric layer thickness. There was a high correlation between the increase in steric layer thickness and dextran solution hydrodynamic diameter suggesting that there was minimal change to dextran structure upon attachment.
[1] D.J. McClements (2004) Curr. Opin. Colloid Interface Sci. 9, 305.
[2] D.J. McClements, Food Emulsions: Principles, Practice and Techniques, 3rd Edition (2004), CRC Press, New York.
[3] E. Dickinson (1997) J. Dairy Sci. 80, 2607.
[4] D.G. Dalgleish, S.E. Friberg, K. Larsson and J. Sjoblom (Eds.), Food Emulsions, 4th Edition (2004) Dekker, New York.
[5] S. Tcholakova, N.D. Denkov, D. Sidzhakova, I.B. Ivanov and B. Campbell (2005) Langmuir 21, 4842.
[6] L. Jimenez-Castano, R. Lopez-Fandino, A. Olano and M. Villamiel (2005) Food Chem. 93, 689.
[7] A. Kato (2002) Food Sci. Technol. Res. 8, 193.
[8] C.A. Dunlap, G.L. Côté (2005) J. Agric. Food Chem. 53, 419.
[9] M. Hattori (2002) Food Sci. Technol. Res. 8, 291.
[10] ] L. Jimenez-Castano, M. Villamiel, P.J. Martin-Alvarez, A. Olano and R. Lopez-Fandino (2005) Food Hydrocolloids 19, 831.
[11] C.A. Dunlap and G.L. Cote (2005) J. Agric. Food Chem. 53, 419.
[12] D.G. Dalgleish (1996) Food Res. Int. 29, 541.
[13] K.D. Caldwell, J. Li, J.T. Li and D.G. Dalgleish (1992) J. Chromatogr. A 604, 63.
[14] Dynamic Light Scattering: An Introduction in 30 Minutes, Technical Note
[15] T.J. Wooster and M.A. Augustin (2006) J. Colloid Interface Sci. 303, 564.
[16] Y. Hemar and D.S. Horne (1998) J. Colloid Interface Sci. 206, 138