Prepared by L. Bergman[1], J. Rosenholm[1], A.-B. Öst[2], A. Duchanoy[1], P. Kankaanpää[2], J. Heino[2] and M. Lindén [1] - [1]Center for Functional Materials, Department of Physical Chemistry, Abo Akademi University, Porthansgatan 3-5, FIN-20500 Turku, Finland. [2] Department of Biochemistry and Food Chemistry, University of Turku, FIN-20014 Turku, Finland.
Nanoparticles have been used in many biological applications including fluorescent markers and drug delivery [1-3]. Their size allows them to cross physiological barriers and access different tissues, followed by an efficient cellular uptake and intracellular internalization [2]. Previous studies have shown that the efficiency of cellular uptake decreases with increasing particle size [4,5] with particles having diameters of 100 to 200nm or smaller displaying the highest uptake [5,6]. Positively charged particles are also beneficial because cell membranes are normally negatively charged.
Silica nanoparticles are biocompatible, chemically inert under a wide range of conditions and can be produced with a tunable particle size and pore structure [7,8]. Silica is negatively charged over the pH range of biological interest, with an isoelectric point (IEP) in the range of 2 to 3.
This application note summarizes results of electrokinetic studies of silica nanoparticles with various surface functionalities relevant to biological systems. The zeta potential of silica can be readily controlled by the attachment of various functional groups. This is important for both a biological activity perspective and for preventing particle agglomeration of electrostatically stabilized suspensions. This is especially important under physiological conditions where the electrolyte concentration is high, resulting in decreased electrostatic repulsion between particles. Surface modification of the particles will influence their zeta potential and could lead to pronounced agglomeration even if the original particle suspensions were stable.
Monodisperse silica particles of 240nm diameter (determined from SEM) were modified with the cationic polymer polyethyleneimine (PEI). These particles were then further modified with either succinic acid or glutaraldehyde. These surface-functionalized silica particles were further conjugated to the protein streptavidin, either native or labeled with a fluorophore. Streptavidin Alexa Fluor 555 conjugate (Invitrogen Molecular Probes) and streptavidin DyLight 549 fluorophore (Pierce Biotechnology, Rockford) were used. Further details of the preparation methods used can be found in the literature [9].
Dynamic light scattering and zeta potential measurements were performed on a Zetasizer Nano ZS instrument at 25°C. The Zetasizer Nano uses a 4mW He-Ne laser operating at a wavelength of 633nm. Electrokinetic titrations were performed with the Zetasizer Nano coupled to a multipurpose titrator MPT2. The zeta potential was measured as a function of pH by titrating with 0.1 or 0.5M HCl and NaOH at 25°C. The samples were suspended in deionized water or a saline solution with the same ionic strength as PBS buffer and dispersed by sonication. The measured electrophoretic mobilities were converted into zeta potential using the Smoluchowski approximation.
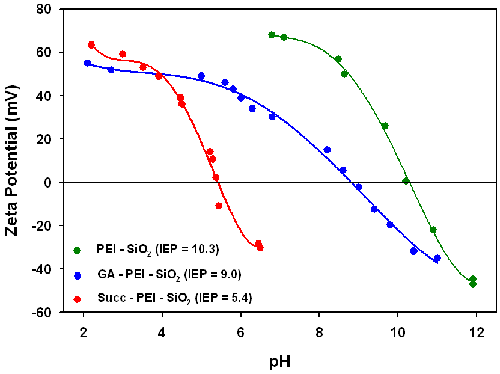
Figure 1 shows the electrokinetic titration curves for the PEI-functionalized silica particles (PEI-SiO2) measured in water. The isoelectric point (IEP - point of zero zeta potential) of the PEI-functionalized silica was 10.3, which is very different to the value of around 2 for non-functionalized silica particles. This much higher value obtained for the PEI-functionalized particles originates from the presence of amino groups on the surface, which are virtually fully protonated at pH values below 9. The fact that the PEI-functionalized particles do show an IEP provides evidence for the presence of remaining surface silanols on the particle surface. The zeta potential of the PEI-functionalized particles is around +60mV in water at neutral pH making them easily dispersible.
|
Modification of the PEI-functionalized particles with glutaraldehyde (GA-PEI-SiO2) results in the IEP reducing from 10.3 to 9.0 indicating successful attachment of glutaraldehyde to a fraction of the amine groups. Since the aldehyde group is not a dissociable group, it will not contribute to the zeta potential. However, its presence decreases the number of the effective amino group content of PEI upon glutaraldehyde modification leading to an increase in the relative concentration of negatively charged silanol groups as compared to positively charged amino groups on the particle surface.
The zeta potential versus pH curve measured for the succinylated PEI-silica particles (Succ-PEI-SiO2) shows that the IEP dropped markedly from 10.3 to 5.4 after succinylation. This provides evidence for the successful introduction of carboxylic acid groups. The pKa of succinic acid is 4.2 so this IEP of 5.4 is higher than expected and suggests that some amine groups still remain in the PEI layer after succinylation causing a shift in the IEP towards a higher value. The high zeta potential values at lower pH also provides evidence for the presence of amino groups remaining from the PEI function.
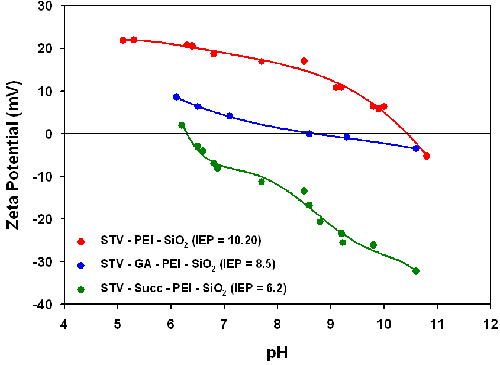
The influence of pH on the zeta potential of all streptavidin conjugated particles in both water and a saline solution (0.154M NaCl + KCl) was also investigated and the results are summarized in Table 1. The conjugation of Alexa555-labelled streptavidin with either GA-PEI-SiO2 or PEI-SiO2 decreased the IEP in water from 9.0 to 6.1 and from 10.3 to 9.3 respectively. Streptavidin has an IEP of 5 to 6 and therefore the conjugation onto the nanoparticles reduces the IEP of both samples. The linking of streptavidin to the PEI-functionalized particles consumes amino groups, while amino groups in the streptavidin are used when linking streptavidin to GA-PEI-SiO2 nanoparticles. In addition, the pH-dependent charging of the Alexa555 fluorophore could also have an influence on the zeta potential. For the Alexa555-streptavidin-PEI-SiO2 particles, the zeta potential values exceed +40mV at pH values below 8 in water, suggesting that these particles are electrostatically stabilized in suspension. The IEP value for the Alexa555-streptavidin-GA-PEI-SiO2 particles is close to 6 and these particles should therefore not form stable suspensions in water. For Alexa555-Succ-PEI-SiO2 particles, a slight increase in the IEP from 5.4 to 5.9 was observed. The IEP of these particles is also very close to the pH of water, suggesting flocculation would be predicted in water if the suspensions are electrostatically stabilized.
| Glutaraldehyde | Succinic Acid | PEI | |
|---|---|---|---|
Stv-Alexa555 in water | 6.1 | 5.9 | 9.3 |
Stv-Alexa555 in saline solution | 7.1 | 6.2 | 8.2 |
Stv in saline solution | 8.5 | 6.2 | 10.2 |
Stv-DyLight549 in water | - | - | 7.9 |
Stv-DyLight549 in saline solution | - | - | 6.8 |
Confocal fluorescence microscopy studies was used to study the flocculation behavior of the differently fluorescently labeled streptavidin particles in water. Virtually no flocculation was observed for the Alexa555-streptavidin-PEI-SiO2 suspensions which correlated with the high positive zeta potential at pH 6 for this system. For the Alexa555-streptavidin-GA-PEI-SiO2 and Alexa555-Succ-PEI-SiO2 nanoparticles, strong agglomeration was observed in agreement with their low zeta potential values.
Table 1 also contains IEP results obtained for both labeled protein-particle complexes and non-labeled streptavidin-particle complexes in saline (154mM NaCl + KCl). The corresponding zeta potential versus pH titration curves are shown in figure 2. The IEP values obtained for the Alexa555-streptavidin -PEI-SiO2 particles in saline (8.2) are slightly different to the values obtained in pure water (9.3). This is probably due to the electrolyte effects on the effective pKa values of the different ionizable groups present in the different systems. The zeta potential values are well below 30mV in all cases at pH 7.4, which lead to agglomeration of the particles under these conditions. The IEP of streptavidin-PEI-SiO2 particles is 10.2 and the zeta potential at pH 7.4 is around 20mV suggesting that this system could be fairly stable even under high ionic strength conditions.
|
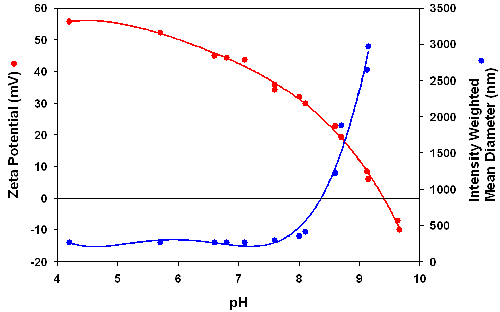
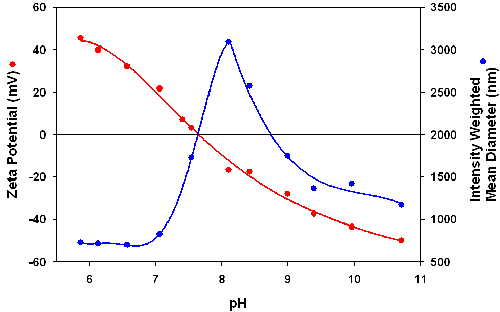
The influence of the fluorophore used on the zeta potential of the particles was studied by comparing the results obtained with DyLight 549 labeled streptavidin with those for the Alexa555-streptavidin-PEI-SiO2 particles. The zeta potential and intensity mean diameters were measured as function of pH and the results are shown in figures 3 and 4. The Alexa555-streptavidin-PEI-SiO2 suspension remains stable up to a pH of about 8 and correlates well with a high positive zeta potential of +30mV or higher in this pH range (figure 3). The DyLight 549-streptavidin-PEI-SiO2 particles have an IEP around 7.9 and agglomerate strongly at pHs higher than about 6.5 (figure 4). These results indicate that the charging of the fluorophore has a strong influence on suspension stability.
|
|
Suspension stability is important in many bioapplications such as drug delivery and cell targeting. The results presented in this application note show that the stability of the suspension is affected by the linker chemistry applied for surface functionalization related to targeting and imaging, by the zeta potential behavior of the biological molecules, and by any additional function introduced, such as a fluorescent dye.
PEI-functionalized silica particles are promising candidates for biological applications due to their high positive zeta potential even under high ionic strength conditions, provided that the introduced additional surface functions do not decrease the IEP of the particles to values close to neutral.
[1] Y.-S. Lin, C.-P. Tsai, H.-Y. Huang, C.-T. Kuo, Y. Hung, D.-M. Huang, Y.-C. Chen and C.-Y. Mou (2005) Chemistry of Materials 17, 4570-4573.
[2] J.K. Vasir, M.K. Reddy and V.D. Labhasetwar (2005) Current Nanoscience 1, 47-64.
[3] P.Sharma, S.Brown, G. Walter, S.Santra and B. Moudgil (2006) Advances in Colloid and Interface Science 123-126, 471-485.
[4] J. Rejman, V. Oberle, I.S. Zuhorn and D. Hoekstra (2004) Biochemical Journal 377, 159-169.
[5] M.M. Amiji (2007) Drug Delivery 53-56, Touch Briefings, London.
[6] I.I. Slowing, B.G. Trewyn, S.Giri and V.S.-Y. Lin (2007) Advanced Functional Materials 17, 1225-1236.
[7] C. Barbé, J. Bartlett, L. Kong, K. Finnie, H. Q. Lin, M. Larkin, S. Calleja, A. Bush and G. Calleja (2004) Advanced Materials 16, 1959-1966.
[8] T. Schiestel, H. Brunner and G.E.M. Tovar (2004) J. Nanoscience and Nanotechnology 4, 504-511.
[9] L. Bergman, J. Rosenholm, A.-B. Öst, A. Duchanoy, P. Kankaanpää, J. Heino and M. Lindén (2008) J. Nanomaterials ID 712514.