Flow injection polymer analysis (FIPA) is a fast, precise and accurate analytical tool for routine process and quality control measurements. This technique provides quick and easy simultaneous measurements of the weight average molecular weight (MW), the weight average intrinsic viscosity (IVW), the weight average hydrodynamic size (RH) and the composition, %Polymer for liquid process/production/research samples. In FIPA, concentration can be calculated so the solutions need to be prepared only at an approximate concentration. In this application communication we show how this technique can be used for a solvent like HFIP, which is very expensive and hazardous for the user, but is an excellent solvent to dissolve polyesters like (PET, PBT) and polyamides (nylon 6 and nylon 6,6). Due to the short analysis time it is possible to make significant savings in HFIP costs and give fast feedback to the plant about the results obtained.
Commercially available PET samples having a nominal Mw were used. The samples were injected sequentially and analysed for their molecular weights and intrinsic viscosity. In this case the samples were prepared to a known concentration, therefore the software was not used to calculate the sample concentration but to verify that 100% of the sample eluted through the FIPA column. All the concentrations were prepared to about 2.4 mg/mL.
The FIPA system used a Viscotek GPCmax equipped with the standard degasser, isocratic pump and autosampler. The column, a ViscoGEL FIPA column was placed in the isothermal compartment of the Viscotek TDA system which was kept at a temperature of 35 °C. The Viscotek Triple Detector Array (TDA) included the RI detector, the light scattering detector and the differential viscometer detector all in the isothermal compartment of the TDA. The solvent used was hexa fluoro isopropanol (HFIP) with 0.1% KF3Ac.
The software calculated a recovery of 100% (+/- 1%) which meant that all the concentration were prepared to the calculated concentration. It also verified that the entire polymer was dissolved in the solution. The +/- 1% deviation is from the noise in the measured concentration.
|
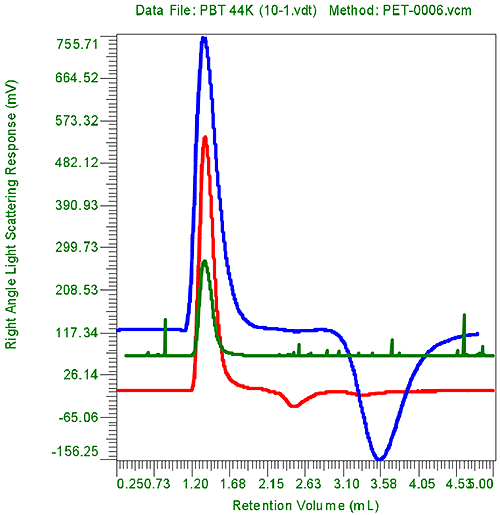
When the repeatability of this measurement is examined we observed that both viscosity and molecular weight measurements can be measured very precisely. Figure 1 shows a typical FIPAgram. In this case the PBT 44K was measured. It should be noted that the volume needed is 5 mL for this chromatogram. In table 1, the injections results are presented. The injection time is also presented showing that injections were made about 10 minute apart.
|
Sample |
Date and Time |
MW (g/mol) |
IVW (dL/g) |
RH (nm) |
|---|---|---|---|---|
|
PET 49K2 |
05/07/2009 10:25 |
47,467 |
0.805 |
8.455 |
|
PET 49K2 |
05/07/2009 10:36 |
48,127 |
0.806 |
8.495 |
|
PET 49K2 |
05/07/2009 10:47 |
48,165 |
0.800 |
8.477 |
|
PET 49K2 |
05/07/2009 10:58 |
48,602 |
0.802 |
8.509 |
|
PET 49K2 |
05/07/2009 11:09 |
48,895 |
0.801 |
8.524 |
|
PET 8K1 |
05/07/2009 11:41 |
5,394 |
0.160 |
2.389 |
|
PET 8K1 |
05/07/2009 11:52 |
5,510 |
0.172 |
2.466 |
|
PET 39K |
05/07/2009 12:03 |
38,855 |
0.748 |
7.716 |
|
PET 39K |
05/07/2009 12:14 |
38,881 |
0.748 |
7.718 |
|
PET 63K5 |
05/07/2009 12:25 |
77,688 |
1.179 |
11.315 |
|
PET 63K5 |
05/07/2009 12:36 |
77,420 |
1.174 |
11.284 |
|
PBT 29K |
05/07/2009 13:09 |
29,449 |
0.830 |
7.285 |
|
PBT 29K |
05/07/2009 13:20 |
29,488 |
0.832 |
7.295 |
|
PBT 37K |
05/07/2009 13:30 |
42,336 |
1.059 |
8.918 |
|
PBT 37K |
05/07/2009 13:41 |
42,484 |
1.065 |
8.943 |
|
PBT 44K |
05/07/2009 13:52 |
51,119 |
1.209 |
9.925 |
|
PBT 44K |
05/07/2009 14:03 |
51,254 |
1.210 |
9.935 |
|
PBT 55K |
05/07/2009 14:14 |
74,571 |
1.608 |
12.377 |
|
PBT 55K |
05/07/2009 14:25 |
75,459 |
1.605 |
12.419 |
|
Nylon 6 |
05/07/2009 14:36 |
85,847 |
2.508 |
15.044 |
|
Nylon 6 |
05/07/2009 14:47 |
84,821 |
2.511 |
14.990 |
|
Sample |
Date and Time |
MW (g/mol) |
IVW (dL/g) |
RH (nm) |
|---|---|---|---|---|
|
PET 49K2 |
05/07/2009 10:25 |
47,467 |
0.805 |
8.455 |
|
PET 49K2 |
05/07/2009 10:36 |
48,127 |
0.806 |
8.495 |
|
PET 49K2 |
05/07/2009 10:47 |
48,165 |
0.800 |
8.477 |
|
PET 49K2 |
05/07/2009 10:58 |
48,602 |
0.802 |
8.509 |
|
PET 49K2 |
05/07/2009 11:09 |
48,895 |
0.801 |
8.524 |
|
Average |
48,251 |
0.803 |
8.492 |
|
|
Standard Deviation |
542 |
0.003 |
0.027 |
|
|
%RSD |
1.1 |
0.3 |
0.3 |
The repeatability is presented in table 2. With 5 consecutive measurements of poly ethylene terephtalate (PET), the molecular weight had a relative standard deviation of only 1.1%. Both the intrinsic viscosity and radius had a relative standard deviation of 0.3%.
FIPA is a fast and accurate way of measuring molecular weight, intrinsic viscosity and hydrodynamic radius. Concentration does not have to be known exactly since it can be calculated through the OmniSEC software. This also means that polymer % can be calculated for each sample. The ratio of IV and MW can give information about branching. For applications with hazardous solvent like HFIP, FIPA is a fast technique that can limit solvent costs and exposure to this solvent. FIPA can easily replace difficult Ubbelohde measurements and since the molecular weight is measured directly via the light scattering detector it avoids calculation via the Mark-Houwink equation which is only valid for molecules of the same structure. The GPCmax solvent delivery system (degasser and pump) includes an auto sampler that can inject from 2 ml vials when only small amounts of sample are available. In this set-up with a flow of 0.5 ml/min it is possible to measure 6 samples in 1 hour which is about 6 times faster than a regular GPC measurement in HFIP.