This application note shows the possibilities of Empyrean for the analysis of gas storage materials, illustrating two examples of interactions with hydrogen.
Such studies are normally performed at large-scale facilities like synchrotrons and neutron sources, or by using dedicated instruments. In this application note we present the results obtained by in situ experiments on H2 storage materials performed on the multipurpose laboratory diffractometer Empyrean in combination with the high pressure heating stage Anton Paar HPC 900.
Data presented in the application note were obtained using Si-based X'Celerator detector. The same experiment can be performed using GaliPIX3D detector featuring 100% efficiency for hard radiation. Within the same measurement time GaliPIX3D provides high quality data with higher intensity (compared to X'Celerator), therefore significantly improving the time resolution of in situ experiments with hard radiation.
This application note shows the possibilities of Empyrean for the analysis of gas storage materials, illustrating two examples of interactions with hydrogen.
The materials for hydrogen storage are normally crystalline and show phase transitions involving a crystallographic symmetry change of a compound upon hydrogen insertion and extraction. These processes can be conveniently studied by means of X-ray diffraction, despite the low scattering power of the solid-state matrix and the fact that hydrogen is essentially invisible to X-rays.
If phase transitions can be monitored during in situ measurements, deeper insights into desorption and/or sorption properties can be reached, and reaction mechanisms can be elucidated. Generally the driving forces of these reactions are temperature and pressure, which require precise control. To follow such reactions in situ, a special sample environment is needed. Additionally, rapid X-ray detection is essential to allow the determination of intermediate phases, onsets of reactions, and reversibility of sorption processes.
Such studies are normally performed at large-scale facilities like synchrotrons and neutron sources, or by using dedicated instruments. In this application note we present the results obtained by in situ experiments on H2 storage materials performed on the multipurpose laboratory diffractometer Empyrean in combination with the high-pressure heating stage Anton Paar HPC 900 [1].
One of the key subjects in materials research for clean energy is the sorption process for storage of gases, such as H2 and CO2. The structural modifications during gas sorption and details of reaction mechanisms can be studied in situ by X-ray diffraction (XRD) in dedicated sample environments.
In situ high-pressure XRD studies mainly belong to the domain of large-scale facilities like synchrotrons or dedicated laboratory instruments. Recent developments of a special sample environment have extended the application area of high-pressure studies to home laboratory systems.
In this application note we present in situ studies of H2 storage materials on the multipurpose laboratory X-ray diffraction system Empyrean, describing two different case studies: the reversible H2 cycling in the system LaNi5 and the H2 desorption during the decomposition of ammonia borane.
While worries for climate change and fossil fuel shortage are constantly increasing, hydrogen has been recognized as a potential clean energy carrier, which, in combination with fuel cells, could contribute to solve the energy problem.
The storage of hydrogen is still a key technological challenge in the implementation of hydrogen fuel cells on a large scale, especially in automotive applications. In principle, hydrogen can be stored in its elemental form, either as a gas or as a liquid, but with efficiency and safety issues. The alternative is the chemical storage of hydrogen, which makes use of a material with low molecular weight (to decrease the storage mass), able to absorb and desorb with rapid kinetics a large quantity of H2, below a temperature of at least 180° C [2-4].
The following configuration of the Empyrean system was used for the measurements:
| Instrument | Empyrean |
|---|---|
| X-ray tube | Ceramic Mo long fine focus
(High-resolution is also possible) |
| Sample stage | High-pressure heating stage HPC 900 with automatic height adjustment |
| Incident beam optics | Programmable divergence slit
(BBHD for Mo is also possible) |
| Diffracted beam optics | Programmable or fixed anti-scatter slit for non-ambient analysis |
| Filter | Beta filter Zr |
| Line detector | X’Celerator in 1D mode
(GaliPIX3D is also possible) |
The High-Pressure Chamber: HPC 900
This chamber has been developed for in situ XRD studies of powder and solid samples under gas pressures up to 100 bar, in the temperature range from 25 °C to 900 °C. The chamber is designed for Bragg-Brentano geometry, especially for operation with hydrogen gas, but other gases can be used as well, such as CO2, O2, N2 and He.
HPC 900 is based on a two-shell design. The inner part is a very compact, high-pressure sample cell, which contains a heater. This part is surrounded by a safety shell, and the internal compartment between the two shells is continuously flushed with inert gas to protect the environment in case of gas leakages.
Figure 1. HPC 900 installed on the Empyrean diffractometer
Figure 2. Top: HPC 900. Bottom: sample holder
Experiments and results
Samples
The temperature sensor is located very close to the sample, allowing accurate temperature measurement.Samples Two hydrogen storage materials were used as case studies to illustrate the possibilities offered by HPC 900: (1) LaNi5 and (2) NH3BH3.
(1) The intermetallic compound lanthanum pentanickel (LaNi5) is one of the first and most studied materials for the reversible storage of hydrogen in a solid matrix [5-8]. LaNi5 has a remarkable affinity for hydrogen, which starts to be absorbed at a pressure of 2 bar at room temperature, to produce LaNi5H6. The main advantages of this compound are the proven reversibility, the mild conditions of H2 sorption and its stability in air, which allows easy handling of the material. On the other hand, its low gravimetric content of H2 (1.0 wt%) makes the compound best suited for stationary rather than portable gas storage applications.
(2) Ammonia borane (NH3BH3) is a crystalline material that was described for the first time in 1955 [9], and has recently received considerable attention as a potential hydrogen storage material [10-12], due to the high hydrogen storage capacity (19.6 wt%) and the good stability in air under ambient conditions. What is more, ammonia borane releases hydrogen rapidly at a relative low temperature of 80 °C, which is beneficial for the automotive industry. However, the sorption process of ammonia borane is irreversible therefore this storage material is ideal for one-shot energy systems.
Sample 1 – LaNi5: H2 cycling in a reversible compound
A feature of LaNi5 is the necessity to be activated at the first cycle of a sorption process. This is done by exposing the sample to hydrogen at temperatures and pressures higher than those needed for the following cycles. In this way hydrogen can penetrate through the protecting layer of La2O3 and La(OH)3 on the surface of a particle [6].Activation and first hydrogenation were performed at 65 bar of H2 and 100 °C. When this step was concluded with the complete transformation of LaNi5 into LaNi5H6, hydrogen was desorbed in vacuum at room temperature, obtaining the pristine LaNi5 phase; two additional full cycles of H2 absorption (in vacuum) were performed at 50 °C. Diffraction data were collected every 2 minutes, at a short angular range where the most intense peaks of LaNi5 and LaNi5H6 are present. A 3D view of the diffraction data is shown in Figure 3. The first step of activation and absorption was very slow and is not shown in Figure 3.
Rietveld refinement [14] was performed in a sequential way on all collected scans by using HighScore Plus software [18], running a user-defined batch. This approach is shown in Figure 4 for the H2 cycles of LaNi5. Le Bail and Pawley profile fitting methods [15,16] are also valid alternatives implemented in HighScore Plus, only requiring space group and unit cell information.
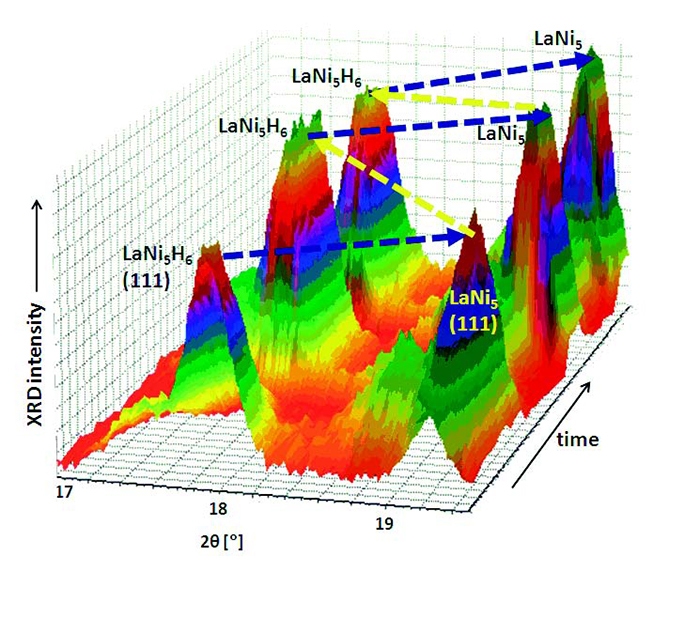
Figure 3. 3D view of the in situ XRD data during the cycles of dehydrogenation of LaNi5H6 (in vacuum) and hydrogenation of LaNi5 (at 5 bar), at 50 °C
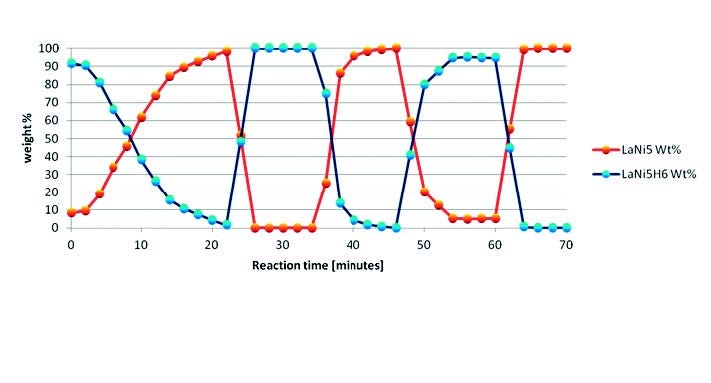
Figure 4. Weight percentages of LaNi5 and LaNi5H6 determined by sequential Rietveld refinement of the in situ XRD data at variable temperature and pressure
Sample 2 – NH3BH3: dehydrogenation of ammonia borane
Ammonia borane was measured in 1 bar of H2, increasing the temperature (4 °C/minute) from 25 °C to 100 °C and collecting XRD data every minute (see Figure 5). Sequential profile fitting was applied in HighScore Plus to obtain the plot shown in Figure 6. It represents the thermal trend of the area of the (110) peak of tetragonal NH3BH3 from room temperature to the decomposition following the dehydrogenation. It was not possible to regenerate any crystalline phase even at 70 bar of H2.
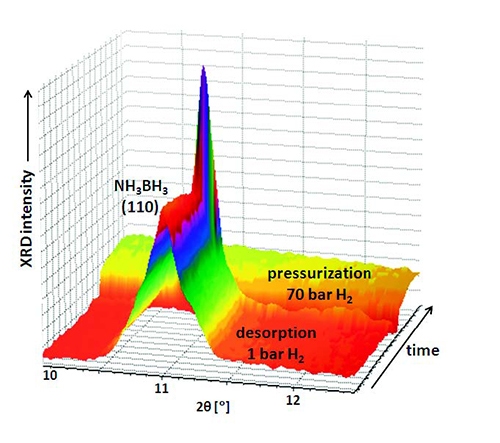
Figure 5. 3D view of the XRD data of the H2 desorption of NH3BH3 in hydrogen atmosphere, and subsequent pressurization to 70 bar H2
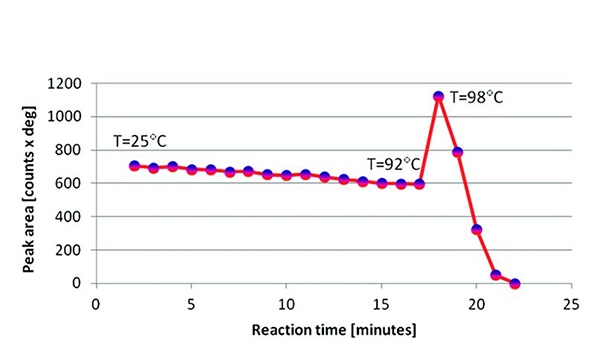
Figure 6. Thermal trend of the area of the (110) peak of tetragonal NH3BH3, from room temperature to complete decomposition
In situ studies of H2 storage materials on the laboratory X-ray diffraction system, the Empyrean, in combination with an Anton Paar HPC 900 sample stage were described for two different compounds. Hydrogen sorption/desorption cycles in LaNi5 as well as decomposition of NH3BH3, accompanied by the release of H2, were monitored. The results demonstrate the high potential of this experimental setup for in-house in situ gas storage experiments. Moreover, due to the wide range of temperature and pressure that can be achieved, the range of gases that can be used and the good thermal control under all operating conditions, the experimental setup can also be used for a large variety of gas-solid interactions.
1. M. Sommariva, H. van Weeren, O. Narygina, J.-A. Gertenbach, C. Resch, A. Pein, V.J. Smith, L.J. Barbour, Mater. Res. Soc. Symp. Spring 2013 Proceedings.
2. L. Schlapbach and A. Züttel, Nature 414, 353 (2001).
3. W. Grochala and P.P.Edwards, Chem. Rev. 104, 1283 (2004).
4. S.i. Orimo, Y. Nakamori, J.R. Eliseo, A. Züttel and C.M. Jensen, Chem. Rev. 107, 4112 (2007).
5. H.H. Van Mal, K.H.J. Buschow and A.R. Miedema, J. Less-Common Met. 35, 65 (1974).
6. W.E. Wallace, R.F. Karllcek and H. Imamura, J. Phys. Chem. 83(13), 1708 (1979).
7. J.-M. Joubert, R. Černý, M. Latroche, A. Percheron-Guégan and B. Schmitt, Acta Materialia 54, 713 (2006).
8. E. MacA. Gray, D.J. Cookson and T.P. Blach, J. Appl. Cryst. 39, 850 (2006).
9. S.G. Shore and R.W. Parry, J. Am. Chem. Soc. 77, 6084 (1955).
10. F F.H. Stephens, V. Pons and R.T. Baker, Dalton Trans. 2613 (2007).
11. Z.T. Xiong, C.K. Yong, G.T. Wu, P. Chen, W. Shaw, A. Karkamkar, T. Autrey, M.O. Jones, S.R. Johnson, P.P. Edwards and W.I.F. David, Nat. Mater. 7, 138 (2008).
12. A. Staubitz, A.P.M. Robertson and I. Manners, Chem. Rev. 110, 4079 (2010).
13. Y. Kim, Y. Kim, S. Yeo, K. Kim, K.J.-E. Koh, J.-E. Seo, S.J. Shin, D.-K. Choi, C.W. Yoon and S.W. Nam, J. Power Sources 229, 170 (2013).
14. H.M. Rietveld, J. Appl. Cryst. 2, 65 (1969).
15. A. Le Bail, H. Duroy and J.L. Fourquet, Mat. Res. Bull. 23( 3), 447 (1988).
16. G.S. Pawley, J. Appl. Cryst. 14, 357 (1981).
17. G. Confazonieri, M. Dapiaggi, M. Sommariva, M. Gateshki, A.N. Fitch, A. Bernasconi, Powder diffraction, Vol 30, No. 2, Sum-1, 565 (2015)
18. T. Degen, M. Sadki, E. Bron, U. Köng, G. Nénert, Powder Diffraction 29, 52, 2014.