With the upsurge in use of portable electronic devices such as tablets and mobile phones, through to the development of environmentally-friendly electronically-powered vehicles, we are experiencing an unprecedented demand for batteries. Developing new, more efficient and more powerful batteries has never been more relevant.
In a previous application note, we discussed the importance of controlling the size of particles used in the manufacture of battery materials. Shape is also an important factor to consider and control, as irregularly-shaped particles not only reduce packing density, but can lead to the formation of a high viscosity electrode slurry.
In this application note, we consider the impact on battery performance of the microstructure of the carbon used to manufacture graphite electrodes, and how the Morphologi G3-ID can aid the development and manufacture of new, more efficient carbon-based battery electrodes.
Figure 1 illustrates the structure of the lithium-ion (Li-ion) battery, the most common battery type used to power portable devices. Typically, a Li-ion battery consists of a graphite anode, metal oxide cathode and a lithium salt in a non-aqueous solvent as the electrolyte.
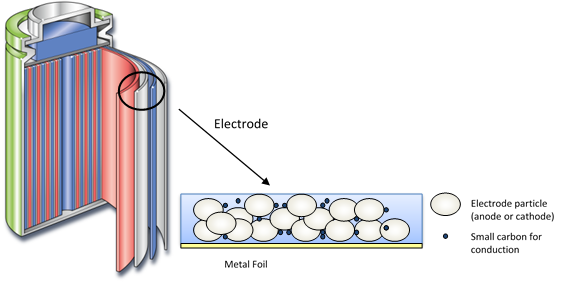
Figure 1: Structure of the lithium-ion battery
The crystallinity, microstructure and micromorphology of the carbon material used to manufacture graphite for Li-ion batteries impacts directly on the batteries’ efficiency, as described below.
To charge a battery, an external power source applies an over-voltage, forcing Li+ ions to embed or intercalate into the layers of graphite of the anode, as illustrated in Figure 2. This intercalation causes the stacking order of the graphite layers to shift and the stacking distance to become larger, increasing the potential energy of the system1. When the over-voltage is removed, the battery will naturally start to discharge from this high energy state: the Li+ ions will migrate out of the graphite layers and be transported via the electrolyte to the metal oxide cathode, which they in turn promote to a higher oxidation state. This charge transfer results in the flow of current and generates the electrical power required to power the device in question.
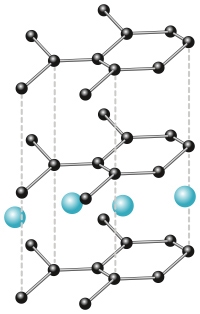
Figure 2: Intercalated graphite
The power of the battery depends on the rate of reaction between the electrodes and the electrolyte, whilst the energy storage capability depends on the volume of the electrolyte and the amount of charge which can be stored in the electrodes. This means that the number of sites into which the Li+ ions can intercalate needs to be maximized, and the Li+ ions must also be able to easily diffuse in and out of the graphite layers so as not to limit the rate at which the charge/discharge reaction can occur2. Thus, they need to have good energy storage, but also be efficient at releasing that energy and being recharged.
The number of sites capable of Li+ accommodation depends heavily on the crystallinity and microstructure of the carbon material1. Highly crystalline graphite will host one Li+ for every C6. Graphite-type structures which store more than one Li+ per C6 are known as “High Specific Charge” (HSC) carbons, while those that support less than this are “Low Specific Charge” (LSC) carbons. Typically, more disordered carbons (like carbon black and coke) are LSC carbons, primarily because the cross-linking carbon sheets in disordered carbon hamper the stacking layer shift, which is required for Li+ intercalation. Higher levels of crystallinity result in the opposite effect.
N.B. This is a generalized description, and the electrochemical behaviour of carbon electrodes does sometimes stray from this rule. Not only has it been shown that very highly disordered carbon can actually have high reversibility capacities, but synthetic non-graphite carbons have also been demonstrated to exhibit HSC2. This may be because the smaller particle sizes involved allow for considerably more Li+ to be stored on the graphite edges and surfaces as well as between layers1.
In pure, crystalline graphite there is only one Raman active mode (the E2g mode) and only one transition observed in the Raman spectrum, at ~1580 cm-1. This is called the G band (Graphite band). However, when not a perfect crystal lattice, the solid state Raman selection rules break down and an additional mode is observed at ~1310 cm-1 - ~1360 cm-1, depending on laser wavelength3. This is known as the D band (Disorder band) and could be due to molecular vibrations which are now Raman active due to the breakdown of crystallinity4. The more disordered the graphite structure is, the stronger the intensity of the D band. Therefore, it follows that the G/D band ratio may indicate the degree of crystallinity of the graphite: it is postulated that the higher the ratio, the more crystalline the material is. Amorphous carbon is not thought to exhibit any Raman transitions in the spectrum measured by the Morphologi G3-ID. Thus, Raman spectroscopy provides a means of distinguishing between different structural forms of graphite.
The Morphologi G3-ID combines automated static microscopy with an integrated Raman probe, allowing the system to fully characterize a sample both morphologically and chemically. The morphological analysis provides a description of the external, physical structure of each particle, and the chemical measurement enables the internal composition of each particle to be elucidated and provides an absolute identification.
In this study, a sample of commercially available graphite was dry dispersed using the Morphologi G3-ID’s automated sample dispersion unit, which enables reproducible and repeatable sample measurement. An automated image analysis was performed on 9000 particles in the sample, generating a detailed morphological profile which was used to direct the subsequent Raman analysis. Raman measurement was performed on a representative sample of the whole particle population: a subset of 500 particles (to ensure the subset of particles was representative of the full population, the size distributions of the particles within each group were compared).
The structure of each particle was assessed based on the Raman spectrum it generated and consequently the degree of crystallinity within the particle was calculated. Each particle was assigned a class based on its degree of crystallinity. It was found that there were three classes of carbon present in the graphite material used for this study: highly crystalline graphite, graphite with a degree of disorder, and amorphous carbon, as illustrated with example particle images in Figure 3.
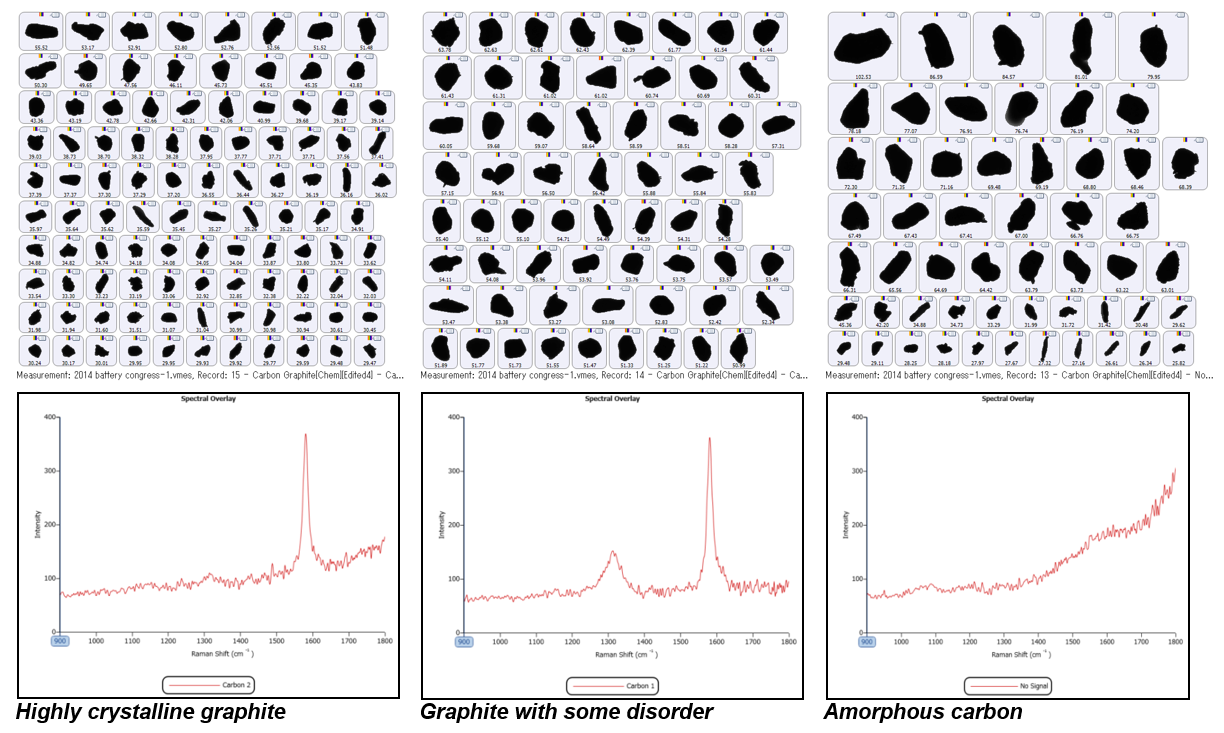
Figure 3: Top - Example particle images for each carbon class; Bottom - Raman spectra associated with the 3 carbon classes
As illustrated in Figure 4, more than half of the carbon sample was found to be composed of graphite with a degree of disorder (Carbon 1). Around 30% of the sample was found to be amorphous carbon, with no Raman signal, and approximately 15% was highly crystalline graphite (Carbon 2).
Figure 4: Composition (by volume) of carbon sample in terms of classes identified by Raman spectroscopy
The morphological data collected for each of the 500 particles analyzed by Raman spectroscopy was then interrogated, as illustrated in Figure 5 and Table 1.
Figure 5: Top – volume-based size distribution of each carbon class identified in the sample; Bottom - HS circularity of the three carbon classes
Sample Name | CE Diameter Mean (µm) | HS Circularity Mean |
|---|---|---|
No Signal (amorphous carbon) | 53.35 | 0.746 |
Carbon 1 (disordered graphite) | 42.21 | 0.811 |
Carbon 2 (crystalline graphite) | 33.99 | 0.792 |
From these results, it can be seen that amorphous carbon typically has a large particle size, with the lowest circularity of the 3 classes. In contrast, the highly crystalline graphite particles, Carbon 2, have the smallest average particle size. Such morphological information about the different carbon classes could be used to quickly identify and filter off carbon particles of interest for applications such as Li-ion battery manufacture, which requires highly-ordered, crystalline graphite. It might also be possible to use this information to determine the source of the commercial graphite: for example, mined carbon tends to contain more crystalline graphite, whereas that formed from the oxidation of acetylene tends to contain more amorphous carbon.
In this application note we have demonstrated how the integrated Raman probe of the Morphologi G3-ID can be used to chemically identify different structural types of carbon within a sample of graphite battery electrode material. This information can be combined with the morphological data from the same technique to assist in the development and cost- and time-efficient manufacture of battery electrodes.
1. M. Winter, J.O. Besenhard, M. E. Spahr, P. Novak, "Insertion Electrode Materials for Rechargeable Lithium Batteries". Advanced Materials, Vol. 10, No. 10, pp. 725-763; 1998.
2. S. Flandrois, B. Simon, "Carbon Materials for lithium-ion rechargeable batteries". Carbon, Vol. 37, pp. 165-10; 1999.
3. Y. Wang, D. C. Alsmeyer, R. L. McCreery, "Raman Spectroscopy of Carbon Materials: Structural Basis of Observed Spectra". Chemistry of Materials, Vol. 2, pp. 557-563; 1990.
4. J. Filik, "Raman Spectroscopy: a simple, non-destructive way to characterise diamond and diamond-like materials". Spectroscopy Europe, Vol. 17, No. 5; 2005.