The diffusion barrier technique is a measurement method that was originally developed for the prevention of protein denaturation during zeta potential measurements with Zetasizer Nano instruments [1-3].
However, the diffusion barrier technique can also be used for any colloidal sample with high conductivity. Zeta potential measurements become more difficult with increasing conductivity due to a number of factors. These include Joule heating, electrode blackening (degradation), sample degradation (aggregation) and electrode polarization. The automatic settings of the instrument try to minimize these potential problems as much as possible. The use of the diffusion barrier technique will further help in minimizing any effects of the application of the field on the zeta potential results obtained.
In the diffusion barrier technique, a small plug of sample e.g. 20µL, is introduced into a folded capillary cell containing the same buffer that the sample is prepared in, and is therefore isolated from the electrodes. The physical distance between the sample and the electrodes means that the sample is protected. This application note discusses the application of the diffusion barrier technique to high conductivity samples through the measurement of various liposome samples.
Liposomes were prepared by sonication as previously described [4]. Three different compositions of anionic liposomes were prepared from dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylglycerol (DPPG) in phosphate buffered saline (PBS). The final concentration of the prepared liposomes was 10 mg lipid/mL PBS as outlined in Table 1.
| Liposome Composition | DPPC (mg) | DPPG (mg) | PBS (mL) |
|---|---|---|---|
| 1 | 29 | 1 | 3 |
| 2 | 27 | 3 | 3 |
| 3 | 25 | 5 | 3 |
Both size and zeta potential measurements were performed on a Zetasizer Nano ZSP at 25°C. The samples were diluted 1 in 100 with PBS for sizing measurements using Dynamic Light Scattering (DLS). For zeta potential measurements, a folded capillary cell was filled with PBS and a 50µL aliquot of liposome sample was carefully inserted into the measurement zone using a gel loading pipette tip (figure 1).
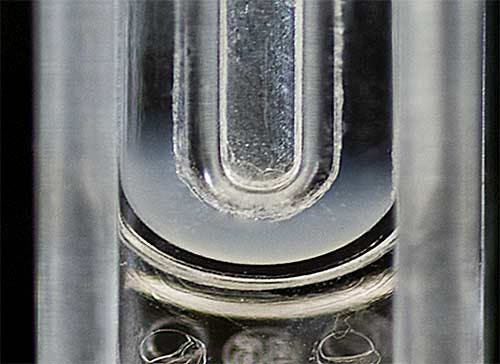
|
The liposome samples measured in this study were prepared in PBS which is a high conductivity dispersant. Measuring the zeta potential of samples prepared in such media is challenging for a number of reasons as previously outlined. Table 2 summarizes the z-average diameters (in nanometers) and zeta potential means (in mV) for the three different liposome compositions measured. The results displayed are the mean values from five repeat measurements together with the standard deviations.
| Liposome Composition | z-Average Diameter (nm) | Zeta Potential Mean (mV) |
|---|---|---|
| 1 | 133.5 (1.35) | -5.8 (0.47) |
| 2 | 77.4 (0.86) | -14.4 (0.43) |
| 3 | 67.1 (0.7) | -19.2 (0.74) |
Both the size and zeta potential results obtained show excellent consistency from repeat measurements. The zeta potential results show the expected trend of the mean values becoming more negatively charged with increasing DPPG content.
The diffusion barrier technique is a method for minimizing the effects of the application of the field on the zeta potential results obtained. Although initially developed for the measurement of protein electrophoretic mobility (or zeta potential), it is also applicable to the measurement of any sample prepared in a high conductivity medium. The repeatable results obtained from the zeta potential measurements in this study confirm this. The other additional benefit of this technique is the low sample volume requirement (down to 20 µL).