This application note describes how the Malvern MicroCal iTC200, which offers the low sample consumption and high throughput needed to drive productivity, has been implemented in a workflow from assay development to lead optimization.
The goal of pharmaceutical research is to modulate the activity of a target such that a therapeutically beneficial response is evoked. A drug is normally only efficacious when bound to and modulating the activity of its physiological target(s). Consequently much early-phase drug discovery is focused on the optimization of a drug candidate’s target affinity and selectivity.
Isothermal Titration Calorimetry (ITC) is widely used for measuring thermodynamic binding parameters for interactions between small molecules and proteins. The simultaneous measurement of binding affinity (KD), stoichiometry (n), free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) provides important information at many stages of the drug discovery process.
The following examples illustrate how the detailed binding information provided by the MicroCal iTC200 system contributes at different stages of drug discovery.
One study involves evaluating peptide binding to target protein during assay development. The goal here is increased understanding of the binding mechanisms of a target protein and a future, potential drug substance. In addition, the resulting data, especially stoichiometry (n) and affinity (KD), can be used to assess the quality and activity of subsequent target protein preparations.
A second application uses ITC as an orthogonal method to examine hits binding to target protein, after primary screen. A positive result from ITC combined with biochemical assay data and data from other biophysical techniques (such as surface plasmon resonance (SPR) and nuclear magnetic resonance (NMR)), helps accurately discriminate between true hits and false positives.
The final study investigates the enthalpic and entropic contributions of the free energy for compounds at the lead optimization stage. The simultaneous measurement of binding affinity (KD), stoichiometry (n), free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) provides important information that supports further development of lead compounds. Enthalpic and entropic contributions to the binding energy provide clues on how a potential drug interacts with the target. This information can be used to build structure-activity relationships and support computer aided drug design.
Although ITC provides this valuable and detailed information in a single experiment, the technology has not always been widely applied in drug discovery because of the significant protein and compound consumption associated with conventional methodologies. The introduction of the MicroCal iTC200 addressed this issue. Compared to older version ITC systems, it provides a seven-fold reduction in sample consumption and two to four times faster equilibrium times, which translates to higher throughput.
All proteins, peptides and small molecule compounds were produced in-house at Hoffmann La-Roche. The MicroCal iTC200 instrument is available from Malvern Instruments. All experiments were carried out at 25°C.
All buffers were degassed prior to use. The sample cell was filled with Bcl-2 (30 µM solution) in 50 mM HEPES, pH 7.4, 100 mM NaCl, 0.5 mM TCEP, and 5% DMSO. The peptides were diluted to a concentration of 250 µM in the same buffer. The injection volumes were 3 µl each, injection time 6 s, and a 150 s delay between each injection. Data was analyzed using MicroCal-enabled Origin™ software (OriginLabs).
|
ITC data, especially stoichiometry (n) and affinity constant (KD), have been used to assess the quality of protein preparations.
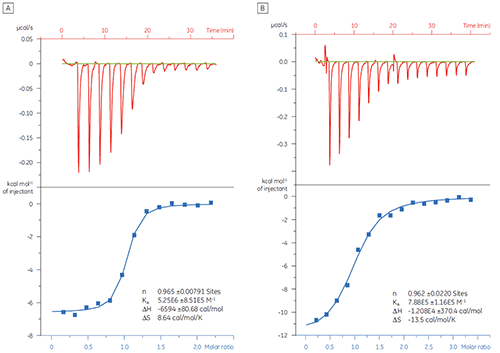
In this example, the interaction of two different peptides with the protein target Bcl-2 (name derived from B cell lymphoma 2) was studied with the MicroCal iTC200 . Results are shown in Figure 1. The binding affinity of the BAD-like (Bcl-2-associated death promoter) peptide for Bcl-2 protein is approximately six-fold stronger than that of the BAX (Bcl-2-associated X protein) peptide.
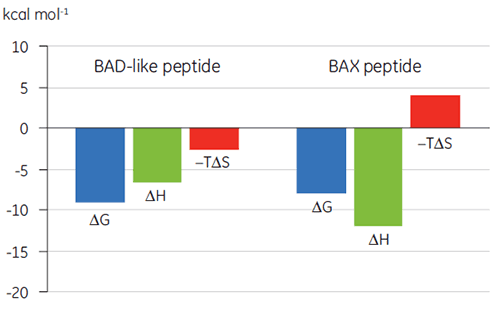
Visualization of thermodynamic parameters in the form of a binding signature plot (Fig 2) makes it easier to see how the enthalpic and entropic components contribute to the overall affinity, represented here by ΔG. These plots reveal that the binding of the BAD-like peptide to Bcl-2 is comprised of polar interactions and hydrophobic interactions, as indicated by the negative or favorable binding enthalpy (ΔH) and entropy factor (TΔS). The binding of BAX involves more conformational changes as indicated by the unfavorable entropy.
|
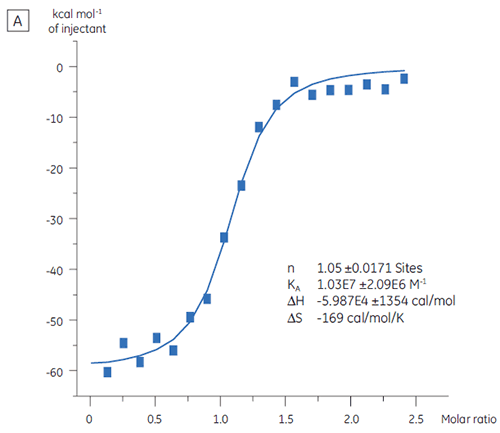
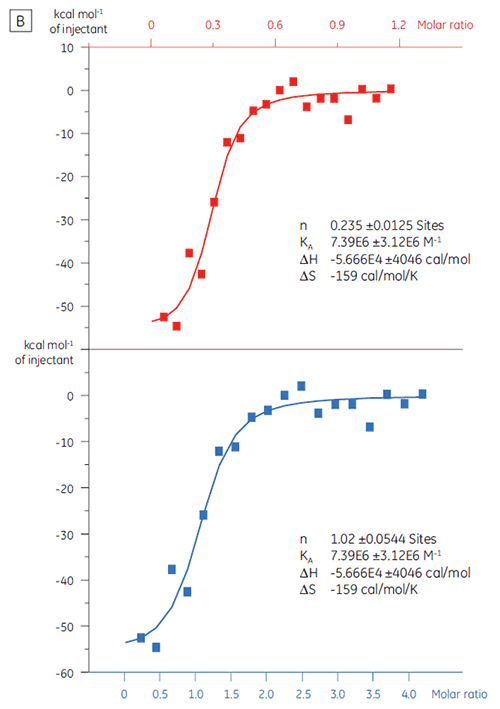
ITC can be used to assess the binding activity level of the target protein before its use in a screening campaign. In this study, two batches of a target protein were compared by titration with a positive control peptide that binds to the protein target with an affinity of 97 nM (Fig 3).
|
|
The result for the first batch, Batch A (Fig 3 A) demonstrates an expected isotherm with a KD of 97 nM and n = 1, indicating a fully active protein. The second batch, Batch B (Fig 3 B) has a KD of 135 nM but n is only 0.23, indicating a protein that is only partially active. Analysis of the same set of data, but with a protein concentration of 2.3 µM instead of 10 µM, gives the same KD value and n = 1. This indicates that 75% of the Batch B protein was inactive. The Batch B protein was rejected for
use in the screening campaign.
It is important to rule out false positives from a screening campaign at an early stage. A 20 µM solution of target protein (TP) was titrated with Compound X (Fig 4). The KD (defined as 1/KA) was determined to 4.9 µM, which correlated well with studies performed with SPR and NMR, thus confirming that Compound X is a true hit and suitable for further studies.
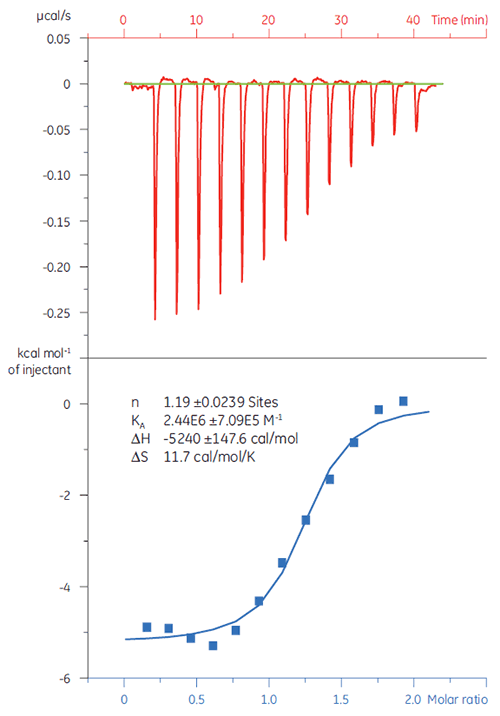
|
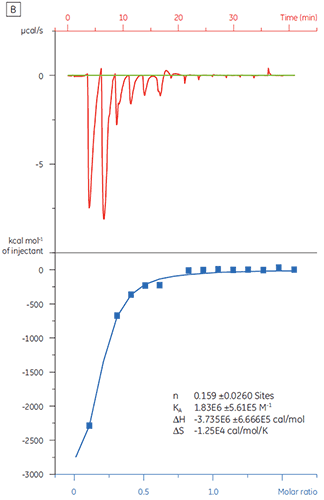
When the same target protein was titrated with Compound Y the results were very different (Fig 5). In the left panel, Compound Y was titrated with TP. The isotherm shows apparent binding affinity of 120 nM but the binding enthalpy was about 1000-fold larger than expected and the stoichiometry value very low (n = 0.01). In the right panel, the same drug candidate was titrated with bovine serum albumin (BSA). Taken together, the results indicate nonspecific activity. Based on these experiments, Compound Y was considered to be a false positive and was rejected from further study.
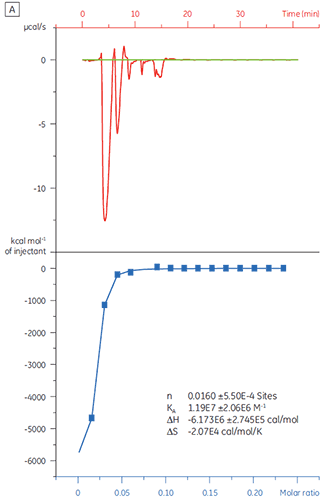
|
|
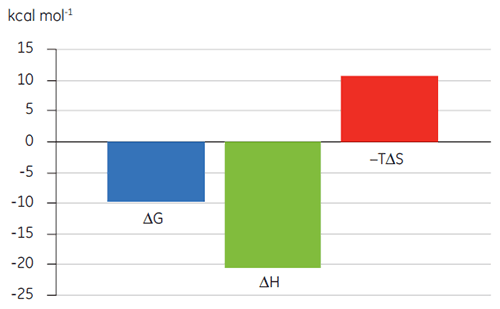
Previous studies of the binding of Compound A to TP suggested a KD of 25 nM. To further characterize the thermodynamics of this interaction, an ITC experiment was performed (Fig 6). A 100 µM solution of Compound A was titrated into a 10 µM solution of TP and the KD value was 65 nM. The binding signature (Fig 7) shows that the affinity is strongly driven by enthalpic interactions but is entropically opposed.
Enthalpic interactions are typically driven by hydrogen bonding and van der Waals interactions whereas favorable entropic binding affinity is derived from hydrophobic interactions. Unfavorable entropy, as in this example, results from an overall decrease in the degrees of freedom of the interacting species relative to the complex. This is always the case when two molecules interact but the magnitude of the effect suggests that the protein has undergone a conformational change or that the ligand is very flexible. Reduction in this unfavorable entropy offers a defined optimization pathway that can be followed using ITC.
It has been shown previously that it is more difficult to optimize the enthalpy than the binding entropy, and that relying heavily on optimizing affinity using only one of these parameters may result in a compound that has poor pharmacokinetic properties (too hydrophilic or too hydrophobic). Taken together, it is better to start with a lead that is enthalpically driven and then to ‘engineer in’ the improved entropy.
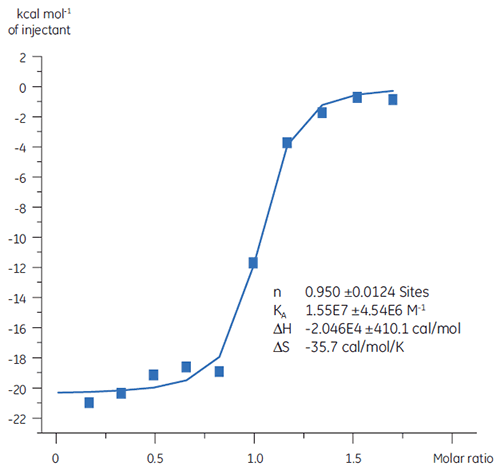
|
|
Using the MicroCal iTC200, the ITC technique has been incorporated into a drug discovery workflow, demonstrating that:
The data were kindly provided by Dr. Lin Gao, Hoffman-La Roche, Nutley NJ, USA.