In this application note, we look at how the NanoSight range of instruments uses Nanoparticle Tracking Analysis (NTA) to determine the state of nanoparticles, and in particular their size, size distribution and concentration in test media prior to nanotoxicological and ecotoxicological studies.
Rapid growth in the development and use of engineered nanoparticles (NPs) continues to run ahead of methodologies for the assessment and management of the risks they may pose. Novel properties resulting from particle size at the nanoscale are the basis to the performance promise of nanomaterials. Even where the toxicology surrounding the product in bulk form is well understood, an automatic "read-across" of properties for the same materials at nanoscale is far from an automatic assumption; these are essentially new materials. Awareness grows that the longer term potential toxic effects of such materials and their potential environmental impact are poorly understood. Consequently, there is intense interest and research activity concerning potential toxicological effects of engineered nanomaterials, accompanied by review of the importance of the nanoscale fractions within existing products and formulations.
Before commencing any nanotoxicological study of suspended nanoparticless, it is imperative to know the state of the nanoparticles used, and in particular their size, size distribution and concentration in an appropriate test media.
Particle size determines diffusion rates, penetration of or exclusion by biological barriers and particulate interactions.
This note discusses the application of Nanoparticle Tracking Analysis (NTA) in the characterization of gold nanoparticles and their aggregates in a biologically relevant fluid. Complementary to classical light scattering techniques, NTA allows nanoparticles to be sized and their concentration measured in suspension on a particle-by-particle basis, enabling high resolution understanding of aggregation. This study looks at the change in size of gold nanoparticles, comparing the case of the standard dispersant solvent (citrate buffer) to a dilute plasma (containing proteins), NIST (2007).
Blood was taken from healthy donors. The tubes were centrifuged for 5 minutes at 800 RCF to pellet the red and white blood cells. The supernatant (plasma) was transferred to labeled tubes and stored at -80 ˚C. Upon thawing, the plasma was centrifuged again for 3 min at 16.1 kRCF to further reduce the presence of red and white blood cells. The supernatant was transferred to a new vessel, taking care not to disturb the pellet.
NIST gold standard nanoparticles of 60 nm diameter were used (NIST reference material 8013). These were stored, prepared and used according to the relevant reports of investigation, NIST (2007). The gold was diluted to a concentration of approximately 108 particles/mL using standard citrate buffer with a pH of 7.19. For the dispersions in plasma, the human plasma was diluted 1:106 in citrate buffer, and 10 µL of gold nanoparticles were diluted with 790 µL of the diluted plasma.
Nanoparticle Tracking Analysis was carried out on a NanoSight LM10. All sample preparation and measurements were carried out at University College Dublin, Ireland. Multiple videos, each 166 seconds long, were recorded and analysed in batch mode to ensure statistical robustness. Given that the plasma is a natural ionic medium, it is noted that the issue of protein aggregation will always be problematic.
Videos of the nanoparticles were recorded (Figure 1), tracked and analysed for size and concentration when diluted in citrate buffer and in human plasma (Figure 2).
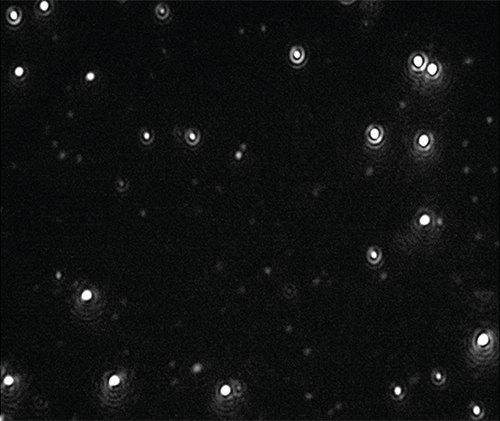
|
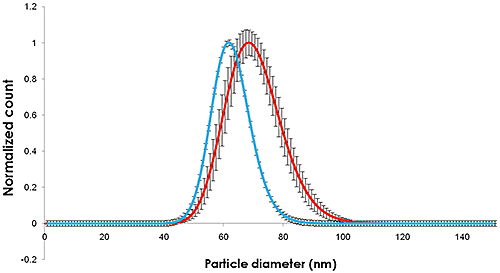
|
The methods for measurement of gold diluted in citrate buffer were as those detailed in the reference materials report, NIST (2007).
NTA can be used to identify both visually and by a concentration measurement that the particles are still monodispersed adding evidence to the hypothesis that proteins in suspension act to coat the nanoparticles (and that the increase in size is not due to aggregation).
The change in size recorded in Figures 1 and 2 show an increase in nanoparticle size of approximately 10 nm, corresponding to a 5 nm thick adsorbed protein layer covering the nanoparticle.
There is a significant and measurable change in particle size distribution of monodisperse gold nanoparticles in the presence of a biological medium such as human plasma. A suitable methodology for the measurement of the thickness of the plasma layer using Nanoparticle Tracking Analysis has been described here.
The breadth and mean of the particle size distribution of the citrate dispersed particles measured by NTA is similar to that determined by NIST. In the presence of plasma, both the particle size and the particle size distribution increase similarly.
NTA offers the ability to measure absolute concentration which may be used to also give a number concentration of the NIST gold nanoparticles. NTA is also ideal for identifying and measuring the concentration of nanoparticle aggregates such as dimers which can be readily differentiated. It thus represents (for the nanoparticles and concentration regimes used here) a useful tool in the existing array of techniques available for bionanoscience and bionanointeractions for engineered nanomaterials.
NTA has been recognized as an analytical method to furnish information about nanoparticle size and, equally importantly, concentration, and is able to do this even in complex sample types of high polydispersity (Montes-Burgos et al., 2010; Lynch, 2008; Montes-Burgos et al., 2007). Methods such as NTA can be considered as one of a number of means by which the environmental impact and potential cellular toxicity of nanoparticles could be studied in the future (Borm et al., 2006; Tenuta, 2008; Tran and Anton, 2009; Kuhlbusch et al., 2010; Hassellöv and Kaegi, 2009; Stolpe et al., 2011).
Recent studies have shown that the complexity of interactions between NPs and environmental matrices is extremely complex and represents a significant challenge in both their quantification and modelling but in which NTA may provide a mechanism of investigation and analysis (Gornati et al., 2009; Hartmann, 2011; Arvidsson et al., 2011; Howard, 2010; Njuguna et al., 2011; Tran et al., 2009).
In a study of the effects of a coating applied to zero-valent nano-iron (nZVI) on early life stage development of three key marine invertebrate species, Kadar et al. (2012) used NTA to study the dissolution of nZVI in seawater showing that the coating helped stabilize the nanometal suspension. Kadar has also studied the effect of NTA-analyzed industrially relevant engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures in which he described subsequent alterations in their growth rate, size distribution, lipid profiles and cellular ultrastructure (Kadar et al., 2012)
Using a 15k oligonucleotide microarray for Daphnia magna, a freshwater crustacean and common indicator species for toxicity, to differentiate between particle specific and ionic silver toxicity and to develop exposure biomarkers for citrate-coated and polyvinylpyrrolidone-coated silver NPs, Poynton et al. (2012) determined the degree of aggregation of silver NPs by NTA prior to studying their toxicity at the genomic level.
In studying the toxicity of zinc oxide nanoparticles to Folsomia candida, Waalewijn-Kool et al. (2012) showed that differences in methods of spiking exposure media to test dispersion size characteristics made little difference to the reproductive capacity of the organism, NTA and TEM both showing the toxicity of the zinc oxide was not related to particle size.
At a cellular level, NTA has proved useful in studying the genotoxicity of cobalt NPs in human peripheral leukocytes (Colognato et al., 2008) and mouse fibroblasts (Ponti et al., 2009). The ability of nanoparticles to cross the human placenta (Wick et al., 2009) and methods to study their effects on other biological barriers have been addressed (Linn et al., 2010) including the transport of silicon dioxide nanoparticles through human skin (Staroňová et al., 2012). Similarly, Filon et al. (2012) reported on human skin penetration of cobalt nanoparticles through intact and damaged skin, suggesting that Co applied as NPs is able to penetrate the human skin in an in vitro diffusion system.
An understanding of the distribution of nanoparticle sizes prior to their introduction to cellular systems for cytotoxological testing is crucial and NTA has proved useful in this regard (Kendall et al., 2009; Patel et al., 2010; Munaro, 2010; Karlsson, 2010). The chemical interactions of nanoparticles of different types with various matrices of biological origin such as serum (Treuel et al., 2010) and organic pollutants (Ben-Moshe et al., 2009) and dithiothreitol (Sauvain et al., 2008) have also been studied.
The toxicological effects of cobalt nanoparticles (Co-NPs) aggregates were examined and compared to those of cobalt ions using different cell lines representing lung, liver, kidney, intestine and the immune system. The overall findings were in line with the hypothesis that the toxic effects of aggregated cobalt NPs are mainly due to cobalt ion dissolution from the aggregated NPs. (Limor et al., 2011).
Arvidsson R, Molander S, Sanden BA and Hassellov M (2011) Challenges in Exposure Modeling of Nanoparticles in Aquatic Environments, Human and Ecological Risk Assessment: An International Journal, Volume 17, Issue 1, 2011, Pages 245 - 262, DOI: 10.1080/10807039.2011.538639
Ben-Moshe T, Dror I and Berkowitz B (2009) Oxidation of organic pollutants in aqueous solutions by nanosized copper oxide catalysts, Applied Catalysis B: Environmental, Volume 85, Issues 3-4, Pages 207-211
Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R and Wood S (2006) Research Strategies for Safety Evaluation of Nanomaterials, Part V: Role of Dissolution in Biological Fate and Effects of Nanoscale Particles, Toxicological Sciences, 90 (1): 23-32
Colognato R, Bonelli A, Ponti J, Farina M, Bergamaschi E, Sabbioni E and Migliore L (2008) Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro, Mutagenesis Advance Access, published online May 25, 2008. and Mutagenesis 2008 23(5):377-382
Filon FL, Crosera M, Timeus E, Adami G, Bovenzi M, Ponti J, Maina G (2012) Human Skin Penetration of Cobalt Nanoparticles Through Intact and Damaged Skin, Toxicology in vitro, http://dx.doi.org/10.1016/j.tiv.2012.09.007,
Gornati R, Papis E, Di Gioacchino M, Sabbioni E, Dalle-Donne I, Milzani A and Bernardini G (2009) In vivo and in vitro Models for Nanotoxicology Testing, in Nanotoxicity (eds S. C. Sahu and D. A. Casciano), John Wiley & Sons, Ltd, Chichester, UK. DOI: 10.1002/9780470747803.ch15
Hartmann NB (2011) Ecotoxicity of engineered nanoparticles to freshwater organisms, PhD Thesis April 2011, Department of Environmental Engineering, Technical University of Denmark
Hassellöv M and Kaegi R (2009) Analysis and characterization of Manufactured Nanoparticles in Aquatic Environments. In: "Nanoscience and Nanotechnology: Environmental and human health implications." (Eds. Lead J.R. and Smith E.) Wiley Interscience, Chapter 6, p 211-266.
Howard AG (2010) On the challenge of quantifying man-made nanoparticles in the aquatic environment, J. Environmental Monitoring, 12, 135 - 142. DOI: 10.1039/b913681a
Kadar E, Rooks P, Lakey C, Whitea DA (2012) The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures, Science of The Total Environment, Volume 439, 15 November 2012, Pages 8-17
Karlsson HL (2010) The comet assay in nanotoxicology research, Analytical and Bioanalytical Chemistry DOI: 10.1007/s00216-010-3977-0
Kendall M, Ding P, Kendall K and Clark H (2009) Nanotoxicology of PM: Particle interactions with lung surfactant polymers, IEH (2009) Proceedings of the Annual UK Review Meeting on Outdoor and Indoor Air Pollution Research, 20-21 April 2009 (Web Report W26), Institute of Environment and Health, Cranfield University, UK, available at: http://www.cranfield.ac.uk/health/ieh
Kuhlbusch TAJ, Fissan H and Asbach C (2010) Measurement and Detection of Nanoparticles within the Environment. Nanotechnology. p229-266
Limor H-A, James KC., Rafi K, Patrice NM, Oded M, Ponti J, Romano R, Rossi F, Golla-Schindler U, Sommer D, Uboldi C, Unger R and Villiers C (2011) Predictive toxicology of cobalt nanoparticles and ions: comparative in vitro study of different cellular models using methods of knowledge discovery from data, Toxicol. Sci. (2011) DOI: 10.1093/toxsci/kfr124 First published online: May 20, 2011
Linn M, Loretz B, Philippi C, Vajda V (2010) Optical characterization of nanoparticles, 8th International Conference and Workshop on Biological Barriers - in vitro Tools, Nanotoxicology, and Nanomedicine, 21 March - 1 April 2010, Saarland University, Saarbrücken, Germany
Lynch I (2008), NanoInteract - dispersion,cell culture standards, protocols, NanoImpactNet WP1 Workshop, UCD, Ireland, 20th June 2008.
Montes-Burgos I, Salvati A, Lynch I, Dawson K (2007), Characterization techniques for nanoparticle dispersion, at European Science Foundation (ESF) Research Conference on Probing Interactions between Nanoparticles/Biomaterials and Biological Systems, Sant Feliu de Guixols, Spain, 3 - 8 November 2007
Montes-Burgos I, Walczyk D, Hole P, Smith J, Lynch I and Dawson K (2010) Characterization of Nanoparticle Size and State Prior to Nanotoxicological Studies, Journal of Nanoparticle Research, Volume 12, Number 1 / January, 2010 DOI: 10.1007/s11051-009-9774-z
Munaro B (2010) Mechanistic in vitro tests for genotoxicity and carcinogenicity of heavy metals and their nanoparticles, Dissertation zur Erlangung des akademischen Grades des Doktors der Naturwissenschaften Eingereicht im Fachbereich Biologie an der Universität Konstanz vorgelegt von June 2009 Konstanzer Online-Publikations-System (KOPS) URN: http://nbn-resolving.de/urn:nbn:de:bsz:352-opus-121714
National Institute of Standards & Technology, Report of Investigation, Reference Material 8013, Gold Nanoparticles, Nominal 60 nm Diameter 13/12/2007.
Njuguna J, Sachse S, Silva F, Irfan A, Michałowski S, Pielichowski K, Kazmina O, Ermini V, Zhu H and Blázquez M (2011) Investigations into nanoparticles generated from nanofiller reinforced polymer nanocomposites during structural testing, Safety issues of nanomaterials along their life cycle, Symposium at LEITAT Technological Center, Barcelona (Spain). 4th and 5th May 2011
Patel D, Kell A, Simard B, Xiang B, Lin HY and Tian G (2010) The cell labeling efficacy, cytotoxicity and relaxivity of copper-activated MRI/PET imaging contrast agents, Biomaterials, DOI:10.1016/j.biomaterials.2010.10.013
Ponti J, Sabbioni E, Munaro B, Broggi F, Marmorato P, Franchini F, Colognato R and Rossi F (2009) Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: an in vitro study in Balb/3T3 mouse fibroblasts, Mutagenesis, Jul 2009; DOI:10.1093/mutage/gep027
Poynton HC, Lazorchak JM, Impellitteri CA, Blalock BJ, Rogers K, Allen J, Loguinov AV, Heckman L and Govindasmawy S (2012) Toxicogenomic Responses of Nanotoxicity in Daphnia magna Exposed to Silver Nitrate and Coated Silver Nanoparticles, Environ. Sci. Technol., DOI: 10.1021/es3001618
Sauvain J, Deslarzes S and Riediker M (2008) Nanoparticle reactivity toward dithiothreitol, Nanotoxicology, 2:3, 121 - 129
Staroňová K, Nielsen JB, Roursgaard MJ, Knudsen LE (2012) Transport of SiO2 Nanoparticles through Human Skin, Basic & Clinical Pharmacology & Toxicology, DOI: 10.1111/j.1742-7843.2012.00873.x
Stolpe B, Lead J, Cole P, Kendall M, Kadar E, Poole J, Whitby C, Colbeck I, Fabrega J and Galloway T (2011) Multimethod characterization of manufactured nanoparticles in toxicity studies, 6th International Conference on the Environmental Effects of Nanoparticles and Nanomaterials, N1.7,The Royal Society, London, 19th-21st September, 2011.
Tenuta T (2008) A Systematic Approach to Assessing Potential Environmental Impacts of Nanomaterials: Nanoparticle Synthesis, Characterization and Impact Assessment, , EPA Scholarship & Fellowship Seminar - 13th November 2008, Hilton Kilmainham Hotel, Dublin 8, Ireland
Tran L and Antón JMN (2009) Nanotoxicology And Engineered Nanoparticle Risk Assessment, Seguridad y Medio Ambiente - Nº 114, p1 de 45
Treuel L, Malissek M, Gebauer JS and Zellner R (2010) The Influence of Surface Composition of Nanoparticles on their Interactions with Serum Albumin, Chem Phys Chem, Volume 11, Issue 14, pages 3093-3099
Waalewijn-Kool PL, Ortiz MD and van Gestel CAM (2012) Effect of different spiking procedures on the distribution and toxicity of ZnO nanoparticles in soil, Ecotoxicology. DOI: 10.1007/s10646-012-0914-3Online First™Open Access
Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, Diener P-A, Zisch A, Krug H F. and von Mandach U (2009) Barrier Capacity of Human Placenta for Nanosized Materials, Environmental Health Perspectives DOI: 10.1289/ehp.0901200, (available at http://dx.doi.org/) Online 12 November 2009