Polymers are used in ceramic processing for a variety of purposes, and the effect on the ceramic slurry will depend on their adsorption behaviour. Zeta potential can be used to investigate the competitive absorption of mixtures of polymers.
In order to obtain highly dense and homogeneous dispersions, polymeric reagents are utilized. Polyelectrolytes, in particular polyacrylic acid and its derivatives, have been widely used as effective dispersants, while binders and plasticizers like polyvinyl alcohol and glycols are commonly employed in the aqueous tape casting of ceramic powders...
The concept of colloidal processing has been successfully applied to the field of structural ceramics and several studies have shown that the inhomogeneities could be minimized and complex shaped products could be made with a high degree of reliability [1-3]. Surface and interface phenomena play an important role in controlling the stability of suspensions [4,5].
In order to obtain highly dense and homogeneous dispersions, polymeric reagents are utilized. Polyelectrolytes, in particular polyacrylic acid and its derivatives, have been widely used as effective dispersants [6,7], while binders and plasticizers like polyvinyl alcohol and glycols are commonly employed in the aqueous tape casting of ceramic powders [8-10].
Recently, zirconia ceramics have gained attention due to their excellent mechanical properties. However, few studies have been directed towards the competitive effects of the different polymeric additives used in ceramic formulations [11,12]. Therefore, this application note details investigations into the surface chemical properties of zirconia suspensions in the absence and presence of poly(ethylene) glycol (PEG), a typical binder and ammonium poly(methacrylate) (APMA), a common dispersant, both individually and in combination.
Zirconia (m-ZrO2) was obtained from S.D. Fine Chemicals Ltd., India. The mean particle size of this sample was measured on a Malvern Mastersizer S and found to be 10.5µm (zirconia).
Poly(ethylene glycol) (PEG) was obtained from Polysciences Inc. USA, while ammonium poly(methacrylate) (APMA) was sourced from R.T. Vanderbilt Inc., USA. The molecular weights of both the polymers were reported to be 10,000 Da.
Electrokinetic experiments were carried out on a Malvern Zetasizer at 25°C. Zeta potential measurements of dilute zirconia suspensions (10mg in 100ml), were determined at different pH values with the background electrolyte (KNO3) concentration kept constant at 10-3M. The pH was adjusted using either nitric acid or potassium hydroxide.
To find out the effect of PEG and APMA on the electrokinetic behavior of the zirconia suspensions, initially 10mg of ZrO2 was dispersed in 50ml of deionized double distilled water. Then 50ml of PEG and/or APMA solution of desired concentration, whose pH was pre-adjusted to the suspension pH value, was added to the ZrO2 suspension and allowed to equilibrate for 5 hours. The zeta potential values were measured and plotted as a function of pH for different polymer concentrations.
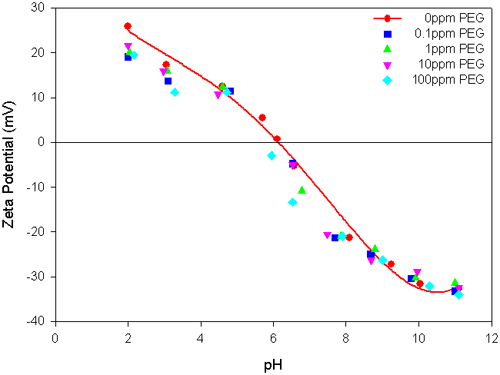
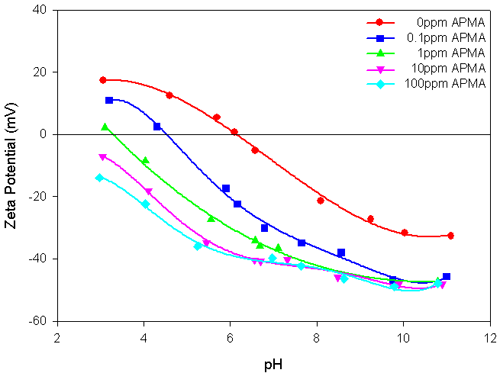
The effect of the addition of PEG or APMA on the electrokinetic behavior of zirconia suspensions is shown in figures 1 and 2 respectively. In the absence of the polymers, the isoelectric point (IEP) of ZrO2 suspensions is located at pH 6.2. The presence of different concentrations of PEG in the ZrO2 suspension has only a marginal effect on the magnitude of the zeta potential values and also there is no shift in the IEP of the suspension (figure 1). This indicates the feeble interaction of PEG with the ZrO2 surface. However, the ZrO2 suspensions in the presence of different concentrations of APMA show significant changes in the magnitude of the zeta potential and also change the position of the IEP (figure 2). APMA, being a negatively charged polymer, shifts the IEP of the system towards the acidic pH region and the magnitude of the zeta potential values becomes more electronegative with the increase in the polymer concentration. This attests to the specific interaction of APMA with zirconia. It is worth noting that at and beyond 0.1ppm APMA concentration, the IEP values are significantly shifted. Further, the zeta potential values become highly electronegative (>-40mV), over a wide pH range of 6 to 11, beyond 10ppm APMA concentration, attesting to good stability of the suspension.
|
|
The electrokinetic behavior of zirconia suspension was also examined in the combined presence of the dispersant and binder, to confirm the dispersing ability of APMA, even in the presence of binder. It was observed that the presence of PEG did not affect the zeta potential values or IEP of the zirconia-APMA system, while trends obtained by the addition of APMA to the zirconia-PEG system closely resembled those of the zirconia-APMA system.
Understanding the interaction between binders and dispersants in ceramic slurries can help determine the optimum conditions for a given ceramic suspension.
In this application note, the IEP of the zirconia sample studied is located at pH 6.2. The zeta potential values and the IEP of zirconia were not altered by the presence of binder (PEG). However, with the increase in concentration of a dispersant (APMA), the magnitude of the zeta potential values of zirconia became more negative and the IEP shifted towards more acidic values, in proportion with the concentration of APMA added.
The electrokinetic properties of the ceramic suspension will influence the degree of aggregation and hence the quality of the final product.
[1] F.F. Lange (1989) J. Am. Chem. Soc. 72, 1.
[2] K.S. Chou and L.J. Lee (1989) J. Am. Chem. Soc. 72, 1622.
[3] S.J. Steadman and R.G. Evans (1990) J. Mater. Sci. 25, 1833.
[4] R.G. Horn (1990) J. Am. Ceram. Soc. 73, 1117.
[5] J.A. Lewis (2000) J. Am. Ceram. 83, 2341.
[6] T.Sato and R.Ruch (1980) Stabilization of Colloidal Dispersions by Polymer Adsorption, Marcel Dekker, New York.
[7] D.H. Napper (1983) Polymeric Stabilization of Colloidal Dispersions, Academic Press, London.
[8] R.E. Mistler (1998) Am. Ceram. Soc. Bull. 77, 82.
[9] E.P. Hyatt (1986) Am. Ceram. Soc. Bull. 68, 637
[10] D. Hotza and P. Greil (1995) Mater. Sci. Eng. A202, 206.
[11] D. Santhiya, S. Subramanian, K.A. Natarajan and S.G. Malghan (1999) J. Colloid Interf. Sci. 216, 143.
[12] K. Ishiduki and K. Esumi (1997) Langmuir 13, 1587.