High-throughput screening in drug discovery
After a drug target has been identified (for instance, a protein associated with a particular disease, identified in genetic studies), the next step is to identify a drug for that protein (that is, a compound that interferes with the target in vivo, curing or alleviating the disease). This phase is called drug discovery, and typically spans 6-10 years.
The drug discovery workflow aims to identify one final drug against a target from thousands of potential compounds. Initially, drug discovery involves identifying promising compounds that interact with the target (hits) from a library of hundreds to thousands of compounds. The binding interaction – often including the binding kinetics – of these hits is then confirmed and they are developed further into leads. The final step is developing the lead into a drug by improving properties such as stability, solubility, or binding.
High-throughput screening (HTS) is the experimental workflow where a large number of compounds are screened for certain traits in a (semi)automated way. In early drug discovery, HTS is often used during hit identification – for example, to screen a library of small molecules for their interaction with a drug target. There is no fixed definition for how many compounds need to be screened to qualify as high-throughput. However, the range of 1,000 to multiple thousands of compounds is typically used.
High-throughput drug screening should significantly reduce the number of compounds to continue with in the workflow, but it also has to identify high-quality hits (avoiding false positives and false negatives). Confirming hits and developing them into leads is time-consuming and expensive. As such, researchers don’t want too many hits, but also don’t want to miss potential drugs by making HTS too stringent.
The early drug discovery workflow can be automated on different levels: HTS in drug discovery spans from the sample preparation by liquid handling devices, through the measurement itself, to analysis and export of the output.
Liquid handling devices take over the pipetting workload required for sample preparation (e.g., transfer, dilution, additions, and incubation). The samples are typically prepared in a multiple-well plate (96-well or multiples of that) and are then transferred to the device that will run and observe the measurement (e.g., measuring the binding kinetics of the compound library to the target).
Depending on the scale and application, the plate transfer between devices is done by robots or manually. Multiple measurements might be done in sequence by one or multiple devices. Ultimately, the data generated from the measurements has to be analyzed, and likely exported and reported.
The WAVEsystem, based on grating-coupled interferometry (GCI) technology, can provide kinetic binding data during early drug discovery. This kinetic data – on the association (ka) and dissociation (kd) rates – provides more information than data on affinity alone, since interactions with the same affinity can have very different kinetics. As such, obtaining data on kinetic binding, rather than just affinity, early on in drug discovery allows you to better select which hits to continue with.
Our proprietary waveRAPID® method can obtain this kinetic data faster than other methods, increasing the throughput and allowing for kinetic screening. The actual throughput will depend on the application: for example, measuring small molecules with fast kinetics typically requires less time than measuring the slower interactions typically found with antibodies.
In addition, waveRAPID® allows for the measurement of binding kinetics from a single well, eliminating part of the sample preparation process (the dilution series).
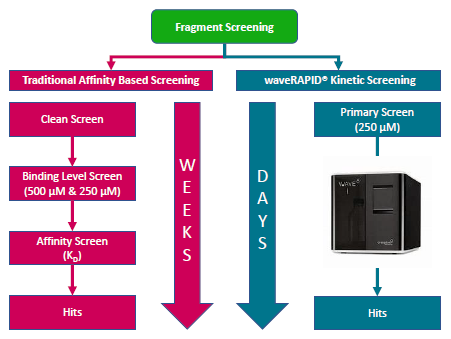
Image right: Traditional affinity-based screening workflow in parallel with waveRAPID® kinetic screening workflow. The waveRAPID® workflow includes the WAVEdelta system, which enables the simple and time-efficient screening of fragments.

HTS is key in accelerating your drug discovery research. The quality of the data created during HTS, which you use to decide which hits to continue with, determines the success of your process.
When carrying out HTS, both avoiding false positives and not excluding false negatives is key: the quantity of compounds identified by HTS for further development affects the effort required to move toward drug development.
Obtaining data on binding kinetics, rather than just affinity, early on allows you to make more informed decisions when identifying and developing these hits.

WAVEsystemNext-generation bioanalytical instruments for drug discovery and life sciences for both industry and academic research |
|
|---|---|