Accurate kinetic measurement of binding events
The constant of dissociation, or dissociation constant, is a kinetic parameter used when measuring intermolecular interactions and binding events between two or more molecules. Where the association rate (ka) describes the rate at which one molecule binds to the other to form a complex, the dissociation rate (kd) describes the rate at which the complex decays, expressed with the unit s-1. Together, these parameters determine the equilibrium dissociation constant, or binding affinity (KD).
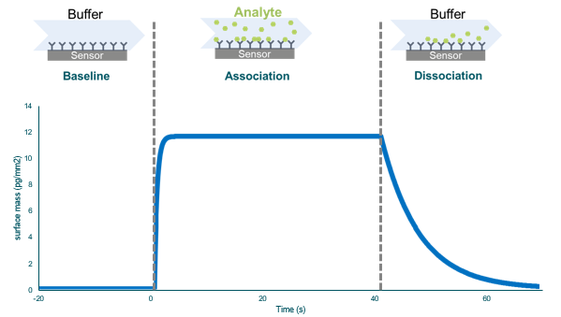
The graph below shows the results from measuring a binding interaction. First, the baseline is established by flowing the buffer past the sensor with an immobilized ligand. After this, the sample (analyte) is flowed past. The analyte associates with the analyte during the association phase, from where the association constant can be calculated. Switching the flow from the sample back to the buffer starts the dissociation phase.
Image right: The resulting curve from a binding interaction measurement (a sensorgram).
Two interactions can have the same affinity (KD), but very different kinetics (ka and kd).
In the example below, the interaction represented by the blue line has fast association and fast dissociation, whereas the interaction represented by the red line has a substantially slower association, and a much slower dissociation.
This results in the same ratio of kd/ka, and therefore the same affinity (KD).
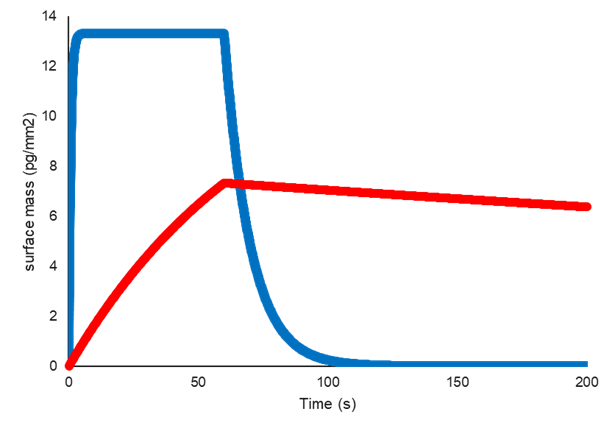
Example of two interactions with the same affinity (KD) but different kinetics (ka and kd)
This means that measuring affinity alone is often not enough to determine which compounds are likely to be worth developing further into a drug. A researcher is likely be looking for a quick interaction to saturation between drug and target, such as in the interaction represented by the blue line.
Within pharmacology, and specifically drug discovery, the dissociation rate is an important parameter for determining a drug’s potential: how long a drug stays in the complex (i.e., drug with drug target) influences its effect on the target, i.e., the effectiveness of the drug. As such, in early drug discovery, potential drugs are often screened and ranked on their off-rate. The dissociation rate (kd), or the off-rate (koff), can be measured with a biosensor.
The WAVEsystem, a Creoptix technology, is an optical biosensor that can measure the dissociation constant as it measures binding kinetics. Its high sensitivity, combined with its compatibility with crude samples and the waveRAPID method that allows higher throughput, makes the WAVEdelta very suited for off-rate screening.

WAVEsystemNext-generation bioanalytical instruments for drug discovery and life sciences for both industry and academic research |
|
|---|---|