Viral vectors are powerful delivery systems for transporting genetic material into cells, leveraging viruses' natural ability to insert genetic code into living cells. Viruses like adeno-associated viruses (AAVs), recombinant AAVs, and lentiviruses are vital in gene therapy, enabling the replacement of mutated genes with healthy ones to treat or prevent diseases.
To unlock their full potential, rigorous characterization is essential to ensure safety, efficacy, and regulatory compliance. Malvern Panalytical offers several biophysical techniques for characterizing viral vectors for gene therapy. These cover critical parameters including particle size, viral titer, surface charge, molecular weight, and thermal and structural stability.
Featured technologies

MicroCal PEAQ-DSC
AAV characterization & rAAV characterization
Small and non-pathogenic, adeno-associated viruses (AAVs) are ideal for vectors for gene therapy.
Malvern Panalytical offers several biophysical tools for AAV characterization. These tools provide faster insights into AAV capsids than common methods such as plaque assays, with the same or even superior precision and repeatability for certain measurements.
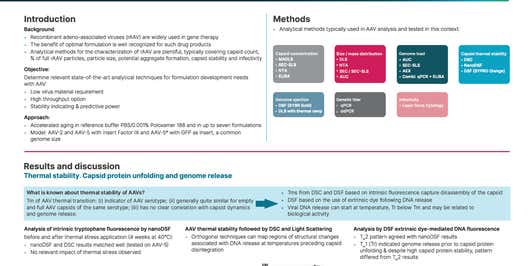
Analytical methods to support formulation development for adeno-associated virus (AAV)

Innovative Solutions Utilized during AAV Characterization

Adeno-Associated Viral Titer in minutes using Nanoparticle Tracking Analysis
Measuring Adeno-Associated Virus (AAV) Concentration
Characterization of Recombinant Adeno-Associated Viruses (rAAVs) for Gene Therapy Using Orthogonal Techniques
Studying Lentivirus Thermal Stability using Zetasizer Advance Ultra and NanoSight Pro
Viral vector stability & storage assessment with NanoSight Pro

Identify VSV-G positive lentivirus with NanoSight Pro F-NTA
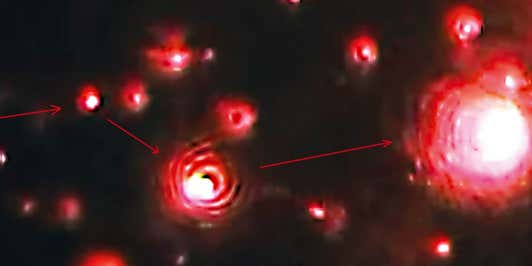
Identify viral vectors with NanoSight Pro F-NTA

Quick viral vector purity assessment with NanoSight Pro

Measuring Lentivirus Size and Titer (Particle Concentration) with the NanoSight Pro

Measuring Lentivirus Size and Titer (Particle Concentration) with the Zetasizer Advance Ultra

Optimizing Lentivirus Storage Conditions using Zetasizer Advance Ultra and NanoSight Pro
MVA characterization
Modified vaccinia Ankara is a weakened strain of the vaccinia virus, which was originally developed as a smallpox vaccine. Modifications mean it cannot replicate efficiently in most cells in mammals, making it ideal for viral vector applications.
When you need data on viral titer, sample purity, thermal stability, and size, nanoparticle tracking analysis and light scattering help you dive deep into your sample’s critical quality attributes.

Quick viral vector purity assessment with NanoSight Pro

Identify viral vectors with NanoSight Pro F-NTA
Why choose Malvern Panalytical?
With a long track record of supporting pharmaceutical organizations bring successful biopharmaceutical products and nano-delivery systems to market, we have the knowhow to help strengthen your analytical workflows and innovate faster than your competition.
From discovery all the way through to quality control, our analytical instruments, supported by an army of problem-solving scientists, can help you turn your therapeutic ambitions into commercial reality.
Explore
Higher Order Structure (HOS)