The global energy and transportation landscape is changing rapidly, with smart energy storage complementing renewable energy. Fuel cells will be an important component of smart energy infrastructure, providing localized energy generation for stationary and mobile applications. In the transportation sector specifically, electric cars powered by hydrogen fuel cells are gaining wider acceptance and will soon have the potential to compete with battery-powered electric cars. Fuel cell cars have the advantage of fast charging, unlike battery-powered cars, which take at least 30 minutes to fully charge.
Moreover, unlike current lithium-ion batteries, fuel cell electrode materials do not use any toxic elements.
Our analytical solutions address many issues in fuel cell development and optimization, such as polymer stability in proton exchange membrane fuel cells (PEMFCs), in situ structural changes in solid oxide fuel cells (SOFCs), and catalyst efficiency. In particular, our instruments can analyze how platinum supported on carbon (Pt black), used as the catalyst for the electrochemical reactions in PEMFC anodes and cathodes, affects fuel cell efficiency. Critical parameters governing a fuel cell’s efficiency for a given Pt loading are Pt particle size, carbon aggregate size, and the catalyst ink formulation governing the catalyst layer’s micro- and macrostructure. Analyzing these factors supports manufacturers to develop the most efficient fuel cells possible.
How can I ensure high specific activity in my fuel cell catalyst?
PEMFCs rely on expensive Pt catalysts, both for hydrogen oxidation reactions (HORs) and oxygen reduction reactions (ORRs). The size of the Pt particles, which are dispersed on a carbon support, directly affects catalytic activity. Smaller, well-dispersed Pt particles have a larger surface area and thus better specific activity and proton conversion efficiency at a given Pt loading.
However, smaller particles may grow bigger upon fuel cell usage at elevated temperatures, due to coalescence via crystal migration or growth via modified Oswald ripening, depending upon the microstructure of the supporting carbon matrix. The microstructure of carbon aggregates also plays an important role in efficient ion transport. Consequently Pt particle size and carbon aggregate size both play an important role in optimizing catalyst activity in fuel cell electrodes.
How can I measure Pt particle size?
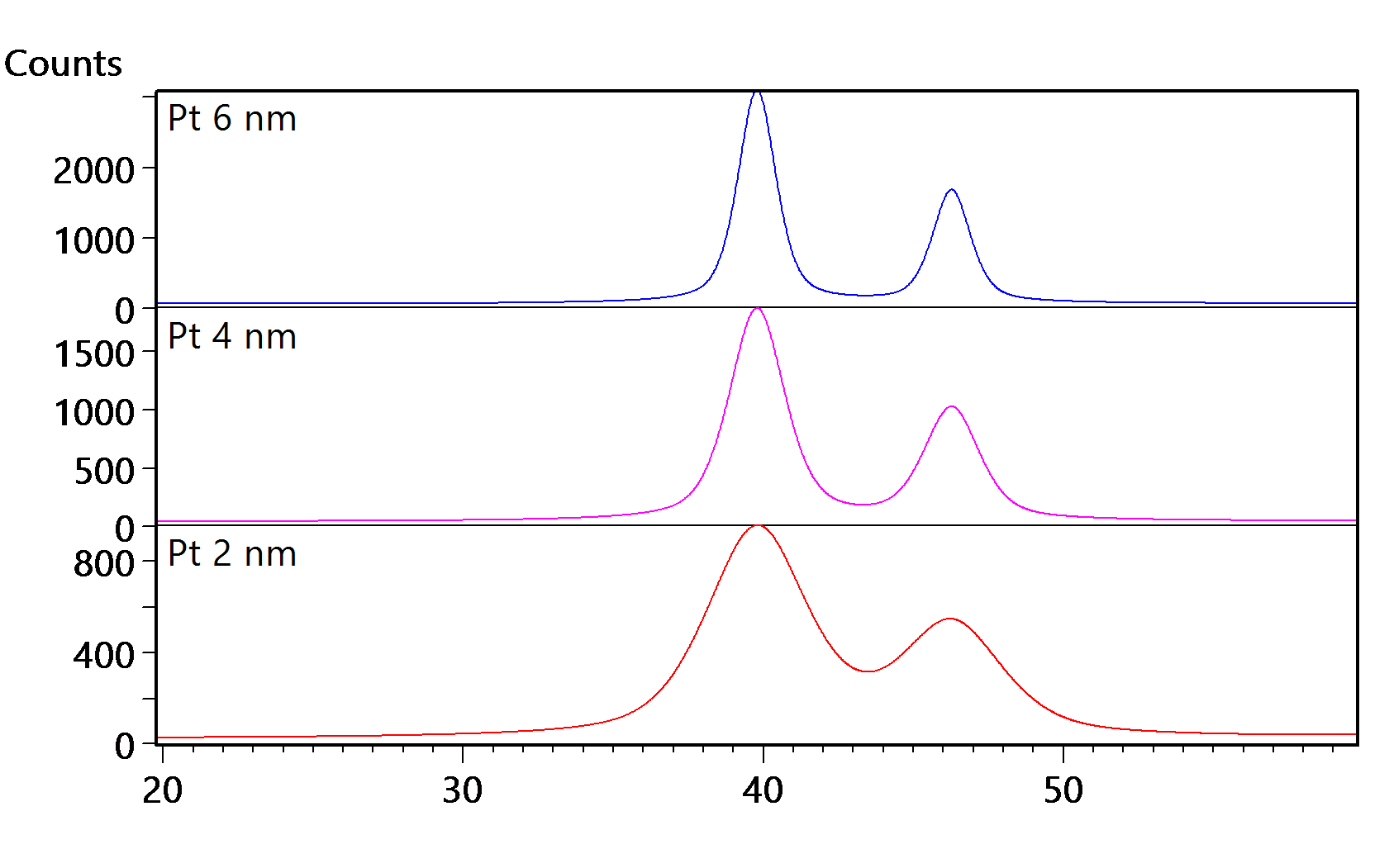
The crystallite size measurements of X-ray diffraction (XRD) can be used to estimate Pt particle size. This is because, in metal nano-particles that typically range from 1-10 nm, crystallite size is likely the same as particle size. This measurement can be accurately done on a compact diffractometer like our easy-to-operate Aeris XRD . Specifically, the Aeris can be used for in situ sintering to understand how Pt particles coarsen with sintering temperature, enabling a more accurate analysis of catalyst activity in PEMFCs.
Another method of directly measuring Pt particle size is small angle X-ray scattering (SAXS). Either SAXS alone, or SAXS and XRD analysis in combination with in situ sintering, can be performed on our Empyrean XRD platform, providing insight into how Pt particle size and coarsening affect fuel cell efficiency.
How can I measure carbon aggregate size?
The carbon particles in fuel cell catalysts can agglomerate to 0.5-5 µm, with highly elongated shapes. Analyzing these shapes helps fuel cell developers to maximize efficiency. To do this, laser diffraction is a non-destructive technique to measure particles of these sizes and compare particle size distribution in different samples. Our Mastersizer range is the leading industry standard in particle size measurement using laser diffraction. Samples can be measured as dry powders or slurry dispersion.
Another method for measuring carbon aggregates is dynamic light scattering (DLS). DLS measures particle size by analyzing the Brownian motion of particles dispersed in a liquid. Larger particles drift slowly while smaller particles drift faster. DLS is very accurate for the 1-1000 nm particle size range of carbon particles, where particles do not sediment due to gravity. Our Zetasizer is the perfect instrument to measure carbon aggregate size in slurry using DLS. Not only this, but the Zetasizer can also measure zeta potential to determine the tendency of particles to form large aggregates.
How can I measure the stability of catalyst ink?
In catalyst ink, Pt particles supported on carbon, along with ionomers, are dispersed in a liquid and usually have a surface charge. Agglomeration of these particles can cause non-uniform coating, leading to high resistance in ion transport. This can be analyzed and prevented by measuring zeta potential, which is related to surface charge. Particles with large zeta potential (over 30mV) repel and are less likely to agglomerate, producing more stable inks.
The Zetasizer , in addition to measuring particle size, can also measure zeta potential. Specifically, it is suitable for measuring electrically conducting samples such as catalyst ink using a specialized cell that measures high-concentration samples. This allows developers to produce more stable inks, enabling more efficient fuel cells.
Our solutions

Aeris
Mastersizer range

Zetasizer range
Further reading



