Polymers are used in ceramic processing for a variety of purposes, and the effect on the ceramic slurry will depend on their adsorption behaviour. Zeta potential can be used to investigate the competitive absorption of mixtures of polymers.
The effect of PEG and APMA on the zeta potential of the alumina samples was studied by preparing dispersions of the ceramic powder in deionized water, adjusting the pH to the desired value and then adding PEG and/or APMA solution of the desired concentration. The samples were allowed to equilibrate for 5 hours prior to the zeta potential measurements.
Polymeric additives of many types of chemical structure are extensively used in ceramic processing for a range of purposes, such as binders, dispersants, plasticizers and lubricants [1,2]. The use of polymers as binders and dispersants are vital for better processing [3,4]. The binders impart strength to the formed products and help in the retention of shape. The dispersants reduce the viscosity of the suspension, facilitating the dense packing of the particles in the product.
Polyethylene glycol (PEG) is one of the important polymers used in ceramic processing as a binder and also as a plasticizer [5,6]. Polyacrylic acid and its derivatives have been widely investigated as dispersants in ceramic slurries to improve their stability [7,8]. The surface chemistry of alumina suspensions in the presence of polyacrylic acid has been investigated. However, there are only a few studies on the adsorption of PEG on alumina surfaces. There is little data on the co-adsorption behavior of polymeric dispersants and binders. This application note investigates the competitive adsorption of ammonium poly(methacrylate) (APMA), a common dispersant and PEG, a typical binder, on alumina. Electrokinetic studies have been carried out on alumina suspensions in the presence of APMA and/or PEG to assess the surface chemical interactions brought about at the solid/solution interface.
α-alumina was obtained from ALCOA, USA. The mean particle size of the sample was measured on a Malvern Mastersizer S and was found to be 0.3µm. Poly(ethylene glycol) (PEG) was obtained from Polysciences Inc. USA, while ammonium poly(methacrylate) (APMA) was sourced from R.T. Vanderbilt Inc., USA. The molecular weights of both the polymers were reported to be 10,000 Da.
Electrokinetic experiments were carried out on a Malvern Zetasizer at 25°C. Zeta potential measurements of dilute alumina suspensions were determined at different pH values with the background electrolyte (KNO3) concentration kept constant at 10-3M. The pH was adjusted using either nitric acid or potassium hydroxide.
The effect of PEG and APMA on the zeta potential of the alumina samples was studied by preparing dispersions of the ceramic powder in deionized water, adjusting the pH to the desired value and then adding PEG and/or APMA solution of the desired concentration, whose pH was pre-adjusted to the suspension pH value. These samples were allowed to equilibrate for 5 hours prior to zeta potential measurements being taken.
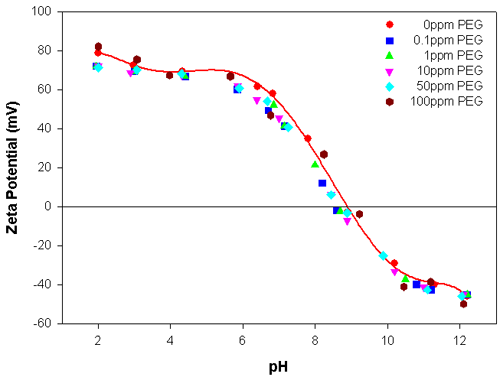
Figures 1 and 2 show the zeta potential results obtained for the alumina sample as a function of pH, with different concentrations of PEG and APMA, respectively. The isoelectric point (IEP) of alumina was found to be at pH 9.2 in the absence of any polymers. Figure 1 illustrates that the addition of PEG only slightly alters the zeta potential values of alumina and there is negligible shift in the position of the IEP. Throughout the concentration range investigated (0.1 to 100ppm), the addition of PEG to the alumina suspension does not appreciably alter the electrokinetic behavior of the suspension. This can be attributed to the weak interaction of PEG with alumina.
|
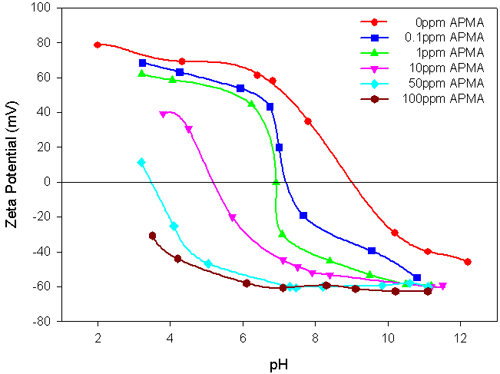
The results in figure 2 show that the negatively charged polymer APMA, significantly reduces the magnitude of the zeta potential values and the IEP of the alumina suspension is shifted towards the acidic region, with the increase in the polymer concentration. This can be attributed to the specific interaction of APMA with alumina. The addition of higher concentrations of APMA enhances the stability of the alumina suspension over a wide pH range of 4 to 11.
|
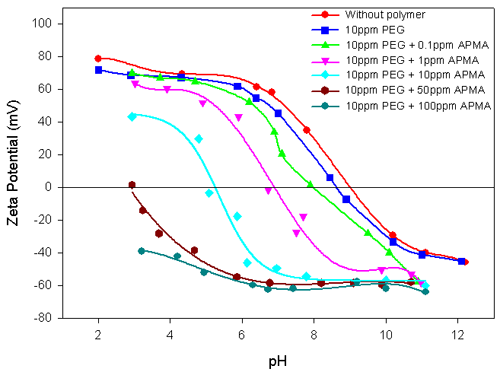
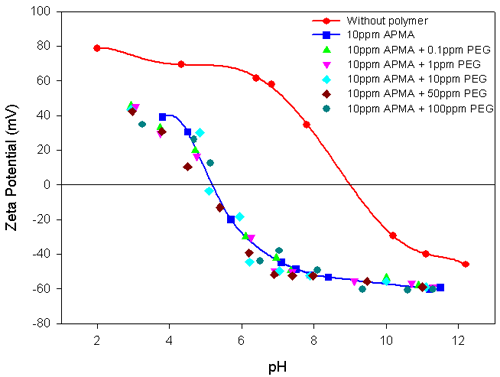
Experiments were carried out on the polymer-interacted alumina suspension in the presence of a second polymer to elucidate their combined influence on the Electrokinetic behavior of the total suspension. Figure 3 shows the Electrokinetic behavior of alumina-10ppm PEG system, with different concentrations of APMA. The striking similarity between this result and the results shown in figure 2 for alumina in the presence of different concentrations of APMA is readily apparent. The stronger specific interactions of APMA with alumina compared to PEG is re-iterated. In contrast, the addition of different concentrations of PEG to alumina-10ppm APMA does not alter either the zeta potential values or the IEP of the system (figure 4). This again attests to the strong interaction of APMA with the alumina.
|
|
The addition of APMA to alumina makes the slurry more electronegative, showing that the polymer is strongly absorbed. The zeta potential is related to the concentration of APMA and the isoelectric point is also shifted to more acidic pH values at higher concentrations. No such changes are observed in the case of the alumina-PEG system.
Electrokinetic studies carried out on alumina suspensions in the simultaneous presence of APMA and PEG essentially follow the trends observed in the case of the alumina-APMA system.
These results suggest that the stability of alumina suspensions is governed more by APMA and that the presence of PEG will not alter the dispersion characteristics of alumina.
The control of the stability of the ceramic dispersion can prevent particle aggregation occurring. The measurement of zeta potential and rheological properties can be used to optimize dispersion conditions for a given ceramic suspension. This will help in producing a final ceramic product with fewer defects.
[1] J.S. Reed, Introduction to the Principles of Ceramic Processing, Wiley, New York, 1988.
[2] M.N. Rahman, Ceramic Processing and Sintering, Dekker, New York, 1990.
[3] R. Moreno (1992) Am. Ceram. Soc. Bull. 71, 1521.
[4] R. Moreno (1992) Am. Ceram. Soc. Bull. 71, 1647.
[5] J. Zheng, J.S. Reed, E.M. Anderson (1994) Am. Ceram. Soc. Bull. 73, 61.
[6] A.S. Barnes, J.S. Reed and E.M. Anderson (1997) Am. Ceram. Soc. Bull. 76, 77.
[7] J. Davies and J.G.P. Binner (2000) J. Eur. Ceram. Soc. 20, 1539.
[8] A. Tsetsekou, C. Agrafiotis and A. Milias (2001) J. Eur. Ceram. Soc. 21, 363.