Characterization of the size and shape of API particles in Metered Dose Inhalation (MDI) formulations are data commonly required to demonstrate in vitro bioequivalence of a generic product to the reference drug. In this application note, we present a study comparing API particle size and shape in a generic and the innovator MDI formulations using the Morphologi® instrument. The comparison is extended to the API granules to investigate the effect of the formulation manufacturing process on the API material.
Over 70 million patients in the world use a metered dose inhaler (MDI) for a variety of lung disorders, such as asthma, chronic obstructive pulmonary disease (COPD), and other conditions characterized by obstruction of airflow and shortness of breath.
Compared to other drug formulations, MDI's have unique differences with respect to formulation, container, closure, manufacturing, in-process and final controls and stability. The composition of an MDI formulation is crucial particularly in defining the physical stability and the performance characteristics of a MDI. Knowledge and information about parameters like particle density, particle size distribution, particle morphology, polymorphic forms of the active, solubility profile etc. are important from a generic manufacturer's perspective for achieving bioequivalence.
This application note presents a study investigating the morphological properties of an active pharmaceutical ingredient (API) in a generic and innovator MDI formulation. This data may be used to support bioequivalence for an Abbreviated New Drug Application (ANDA). By comparing the formulated API particles to the pure granulated API, it was also possible to investigate the impact of the formulation process on the API material. This information can be used to isolate differences in the manufacturing processes of the innovator and generic product.
Both the MDI formulations (Innovator and Generic) were actuated on a microscopic glass slide from a distance of approximately 6 centimeters using the same device. The formulations were composed of the API, ethanol, propellant and oleic acid. Out of these, the propellant and ethanol evaporated during the actuation process, droplets of oleic acid were found in trace amounts and the dispersed active particles were retained on the microscopic glass slide. Morphological characteristics of the particles were investigated by automated image collection and analysis using the Morphologi® by Malvern Panalytical.
Figure 1 presents the overlay of the number weighted circular equivalent diameter (CED) size distribution for the innovator and generic MDI formulations along with example particle images. No significant difference between the two was observed.
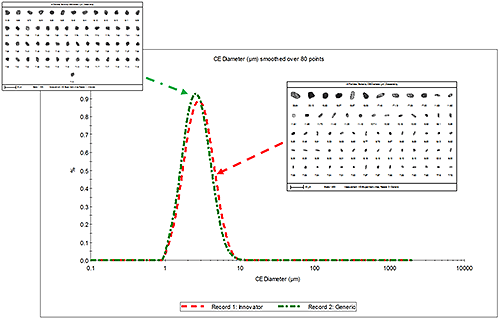
|
However, in terms of particle shape there was considerable difference observed between the two samples. Figure 2 shows the overlay of their convexity distributions, a measure of edge roughness. A convexity value towards 0 indicates a more "spiky" or rough edged particle, conversely a value near to 1 indicates a smooth particle. The lower convexity of the API particles in the innovator formulation indicates a rougher surface texture compared to the API particles in the generic formulation.
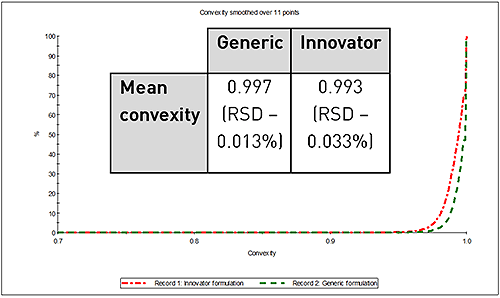
|
Particle aggregates and individual particles were classified on the basis of a morphological parameter known as "Solidity". Solidity is defined as the actual particle area divided by convex hull area of the particle and is very helpful in classifying or even filtering out aggregates from the data set. Figure 3 shows that the percentage of API particle aggregates post actuation was higher in the innovator formulation (65.09%) compared to the percentage of API particle aggregates in the generic formulation (52.96%). This correlates well with the previous observation (figure 2) that the innovator formulation contained particles with rougher edges than the generic sample, since particle aggregates will have rougher edges than primary particles.
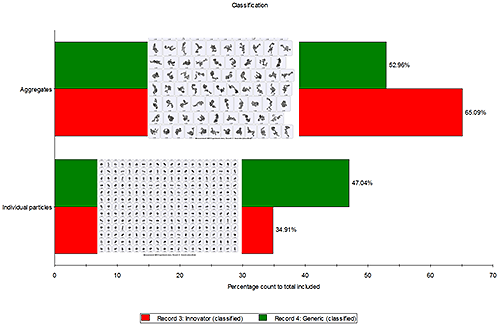
|
Since the same device was used for actuating the samples, the differences observed appears to be related to the API material itself. Further investigation was carried out using Morphologi to find out whether the changes in API morphology was a result of changes in the source API or whether they were introduced during manufacture. For this, the generic API from source was analyzed and the result compared with the generic API in the formulation. Since the source API was in the powder form, it was dispersed using an evaporative technique. The particles were dispersed in solvent and an aliquot of the resulting suspension was spread onto a microscope glass slide. The solvent was allowed to evaporate leaving a thin, well dispersed film of the API particles on the slide.
Figure 4 presents the overlay of the convexity distribution for the source and formulated API particles. The particles in both samples were found to be of similar convexity which suggests the difference in convexity observed between the innovator and generic formulations came from the source API and is not introduced during the formulation process.
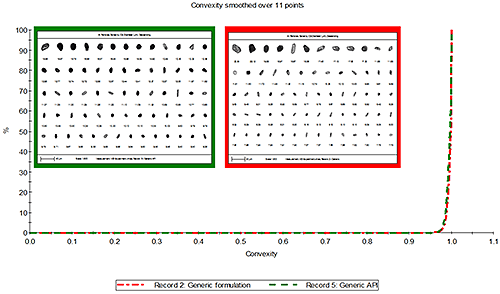
|
The Morphologi was used to analyze and compare the morphological characteristics of an API in an innovator and generic MDI formulations.
This study found that whilst the size characteristics of the API in the generic and innovator formulations were similar, the samples differed in terms of convexity which may be a result of an increased percentage of agglomerates in the innovator formulation. Furthermore, the difference in convexity had its roots in the granulation process of the API rather than the manufacturing process of the formulation.
1. USFDA Draft Guidance for Industry, MDI and DPI drug products. Chemistry, Manufacturing and controls documentation.
2. C Terzano; Metered Dose Inhalers and Spacer Devices. European Review for Medical and Pharmacological Sciences.