Basak Kayitmazer, Paul Dubin, Department of Chemistry, IUPUI Indianapolis IN, USA
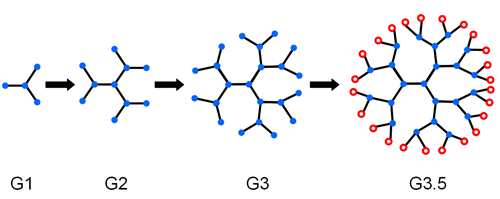
Dendrimers are densely branched molecules of well-defined (spherical or nonspherical) geometry [1]. In colloid science, they are of particular interest due to their model supramolecular structures. Studies have started to focus on a specific kind of these dendrimers, referred as poly(amidoamine) “starburst” dendrimers, due their structural similarity to biomacromolecules [2]. Starburst dendrimers have a core composed of an initiator amino group (##icon1), which is covalently linked to radial interior layers composed of amidoamine (N-CH2-CH2-CO-NH-CH2-CH2-N) units.
The generation (G) is defined by the number of amidoamine layers, as shown in figure 1, where each unit is representative of a complete amidoamine unit. Starburst dendrimers are termed as “full generation” if the outermost generation has amino groups and as “half generation” if the terminal groups are carboxylate groups (see figure 1, where O represents the terminal carboxyl group in a ½ generation dendrimer). Due to the existence of titratable carboxyl and amino groups in starburst dendrimers, investigation of zeta potential of these dendrimers can give a better understanding of globular proteins and enzymes, which also possess carboxyl and amino groups in their primary structures.
|
A G 6.5 starburst dendrimer, composed of 96 amine groups and192 carboxyl groups, was dissolved in 56mM NaCl to a final dendrimer concentration of 1mg/mL. The carboxylated dendrimer sample was a gift of D.A. Tomalia and synthesized according to the methods described elsewhere [3].
The pH dependence of the zeta potential of the dendrimer sample was measured using a Malvern Zetasizer Nano-ZS with a coupled MPT-2 automatic titrator. The pH adjustments were performed across a pH range of 2.6 to 9.3 using 0.3 pH unit increments. The initial sample volume was 8 mL, and the titrants were 0.1M NaOH and 0.1M HCl.
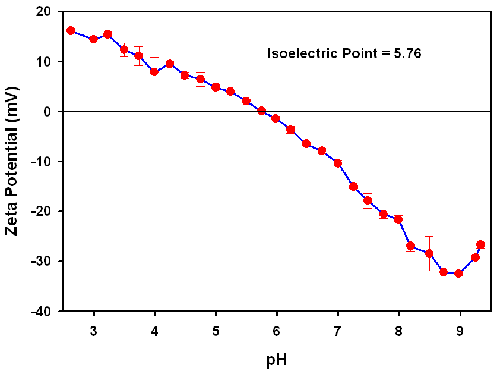
Figure 2 shows the pH titration results for the G 6.5 starburst dendrimer in 56mM NaCl. The isoelectric point is defined as the pH at which the average net charge is zero. Since particles with zero net charge, would also exhibit no translational motion under the influence of an applied electric field, the isoelectric point can also be defined as the pH of zero zeta potential or zero electrophoretic mobility. As seen in figure 2, an isoelectric point of 5.75 is observed for the starburst dendrimer.
|
At pH values greater than 5.76, carboxyl groups are titrated, and the zeta potential becomes more negative as the negatively charged carboxyl groups are neutralized with decreasing pH.
However, the existence of a positive zeta potential in the acidic region of the titration curve (pH < 5.76) is a clear indication of the presence of titratable amino groups.
[1] Advances in Dendritic
Macromolecules (1993) G.R Newkome, Ed; JAI Press: Greenwich CT.
[2] M.F. Ottaviani, F. Montalti, M. Romanelli, N.J. Turro and D.A. Tomalia (1996) J. Phys. Chem. 100, 11033-11042.
[3] Y. Li, P.L. Dubin, R.
Spindler and D.A. Tomalia (1995) Macromolecules 28, 8426-8428.