This application note reports work carried out at the Martin-Luther-University Halle in Germany, which has shown that by using a PANalytical XRD diffractometer fitted with the X’Celerator detector, it is possible to measure the kinetics of the changes in interlayer dimensions in lamellar calcium aluminate chromate hydrate at room temperature in air.
X-ray diffraction (XRD) offers researchers in building materials and those working in environmental monitoring a powerful technique to study phase changes.
Historically, cement has contained typically around 50 ppm of chromium in its hexavalent (Cr VI) state. This arises from the oxidation of traces of naturally-occurring trivalent chromium (Cr III) in raw materials and the use of chromium-containing refractories in the lining of kilns. Cr VI is known to cause allergic contact dermatitis, and has been shown to be toxic and carcinogenic to plants and animals. Cr III is more stable in the environment, and non-toxic.
European Union (and individual member state) regulation now restricts the level of Cr VI in cement to no more than 2 ppm and the industry is taking steps to comply with the new directive (76/79/EEC). Since January 17th 2005, for example, manufacturers in the UK must add a reducing agent to all products to convert Cr VI to Cr III. Unfortunately, this approach offers only a temporary solution as the reducing agent is effective for a limited period.
Other strategies to reduce Cr VI include the re-lining of kilns with non- chromium-containing refractories - a costly and long-term project for most manufacturers - and the immobilization of Cr in the lamellar phases formed during the hydration of Portland cement. This latter approach offers perhaps the greatest promise of a sustainable solution. However, observing and understanding the kinetics of the reactions taking place during phase transitions - a precursor to being able to optimize Cr immobilization - has always been a challenge.
X-ray diffraction (XRD) offers researchers in building materials and those working in environmental monitoring a powerful technique to study these phase changes. However, traditional XRD systems that require long data collection and analysis times do not deliver the speed and resolution necessary to analyze these very rapid reactions Malvern Panalytical’s development of the fast linear solid state detectors X’Celerator and PIXcel now provide the required speed. A Malvern Panalytical Empyrean theta-theta system with solid state detector can be applied highly effectively in this area of research, and measurements can be made in situ.
This application note reports work carried out at the Martin-Luther- University Halle in Germany, which has shown that by using a Malvern Panalytical XRD diffractometer fitted with the X’Celerator detector, it is possible to measure the kinetics of the changes in interlayer dimensions in lamellar calcium aluminate chromate hydrate at room temperature in air.
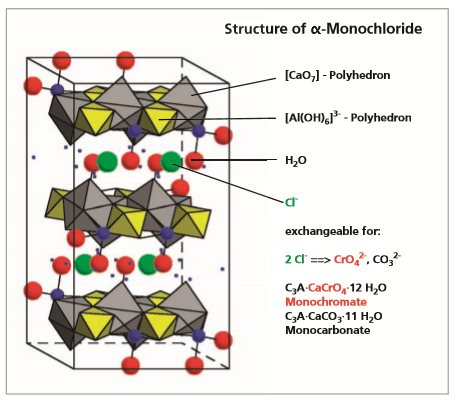
Lamellar calcium aluminate hydrates are products of the hydration of ordinary Portland cement and play an important role in its hardening process. They can also be used as storage minerals to immobilize pollutants.
Reaction Kinetics is the method of describing the rate of a reaction and the velocity with which a reactant, or reactants, undergoes a chemical change. It classifies the speed of reaction and the reaction mechanism. Choosing the correct reaction mechanism allows us to pre-determine the reaction kinetics.
The method of determining reaction kinetics is by observing (quantifying) the substance over a period of time at pre-determined intervals. However, in order to be able to study reaction kinetics, the measurement of the phase reaction has to be quicker than the kinetic change. And, we have to be able to determine the involved phases over the complete concentration range.
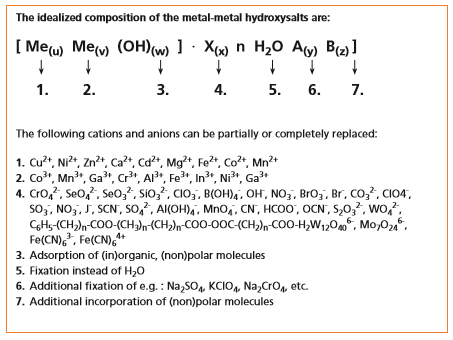
Figure 1: Chemical composition of metal-metal hydroxysalts
Measurements were made using a Malvern Panalytical Theta-Theta diffractometer with X’Celerator detector.
Malvern Panalytical multi-purpose X-ray diffraction systems are ideal for demanding environments and applications. Thanks to the PreFIX concept for fast and accurate changing of optical modules and sample stages, the system can be easily reconfigured from a high-end system for the crystallographic researcher to a ‘work horse’ for routine measurements
The kinetics of changes in interlayer dimensions of lamellar calcium aluminate chromate hydrate at room temperature in air were investigated using lamellar calcium aluminate chromate hydrate monochromate with the idealized formula
C3A·Al2O3·CaCrO4·Ca(OH)2·nH2O. Monochromates were selected from H2O- and CO2-free polyethylene vials and samples were prepared using a drop of CO 2--free distilled H O on a zero background holder in air. The possible ion-exchange and hydration or dehydration of these substances was investigated in this wet atmosphere.
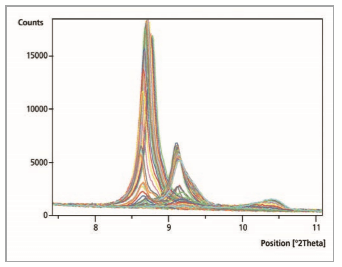
Figure 2 shows a comparison of 150 measurements. Continuous measurements generated results every minute. The normal measurement range was from 7.0 °2θ to 70.0 °2θ. Accordingly, the whole ‘reaction time’ was 150 minutes. The shift in the position of the 100% intensity peak of the chromate containing lamellar phases towards a greater value of 2θ, shows that different ‘reactions’ have taken place.
Figure 2: Comparison of 150 measurement files within the range of 7.5 to 11.0 °2θ
Figures 3, 4, 5 and 6 are diffractograms showing various time steps and accordingly different interpretations of possible reactions. The strongest lines in 2θ of the phases known are:
Additionally there are further solid solutions in this system and investigations into
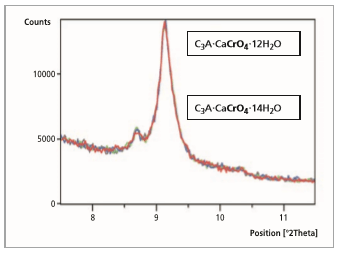
these are described in references [4,9,10]. From the start of the measurement up to a reaction time of 5 minutes, the pure and powdered monochromate C3A·CaCrO4·9H2O (100 % peak = 10.52 °2θ) change to the hydrated phase C3A·CaCrO4·12H2O (100 % peak = 9.07 °2θ) over the course of 1 minute. Figure 3 shows the development of the hydrate phase C3A·CaCrO4·14H2O (100 % peak = 8.63 °2θ)
After a reaction time of 5 minutes (Figure 3) the hydrate stage C3A·CaCrO4·12H2O (100 % peak = 9.07
°2θ) changes completely to the hydrate stage C3A·CaCrO4·14H2O (100 % peak = 8.63 °2θ). An ion exchange of CrO 2- through OH- in the interlayer is possible, but cannot be proved.
Figure 3: Comparison of 5 measurement files, reaction time from 0 to 5 minutes
After a reaction time of 10 to 20 minutes (Figure 4) an ion exchange of CrO 2- through OH- in the interlayer of the hydrated phase C3A·CaCrO4·14H2O (100 % peak = 8.63 °2θ) is visible. A new characterized phase C3A·1/2Ca(OH)2·1/2CaCrO4·14-18 H2O (100 % peak = 8.51 °2θ) occurs [9].
Figure 4: Comparison of 5 measurement files, reaction time from 5 to 10 minutes
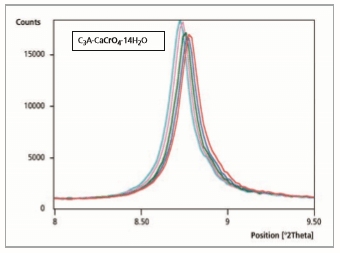
During the drying process on the sample holder, there is a re-exchange from CrO 2- through OH- in the interlayer of C A· 1/2Ca(OH)2 ·1/2CaCrO4 · 14-18 H2O (100 % peak = 8.51°2θ). The chromate-containing phase C3A·CaCrO4·12 H2O (100 % peak = 9.07 °2θ) is formed again after 20 minutes reaction time.
Figure 5: Comparison of 11 measurement files, reaction time from 10 to 20 minutes
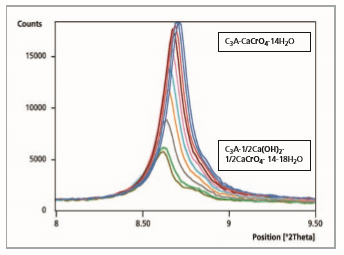
Figure 6: Comparison of 11 measurement files, reaction time after 20 minutes
The Malvern Panalytical Theta-Theta diffractometer with X’Celerator detection technology is a good tool to measure the kinetics of the changes in interlayer dimensions in lamellar calcium aluminate chromate hydrate at room temperature in air. Metric parameters, especially the c0- dimension and the layer distance c’ can be refined. Changes in the c dimension re ect the different substitutions in the interlayers. The ability to make accurate measurements allows for the first time detailed investigation of changes to these structures to understand the related reaction kinetics.
The X’Celerator allows:
Lamellar calcium aluminate hydrates are hydration products in ordinary Portland cement and play a role in the hardening process. Their ability to fix both organic and inorganic ions means they can also be used as reservoir minerals for pollutants. Formation and fixation of pollutants can take place:
In concrete reacting with environmental pollutants
The chemical composition of lamellar calcium aluminate hydrates can be described by the general formulae:
3CaO·A O CaX·nH O and 3CaO·A O CaY nH O
where X= SO4 , CO , CrO4 and Y= OH , C , NO
with the formula C A·Ca(OH) nH O is especially suitable as storage mineral, because of its layered structure. It is widely used to incorporate efficiently e.g. heavy metal compounds, because the OH-group in the interlayers can be exchanged for 1 or 2 anions