Regulatory compliance is essential in the pharmaceutical industry. Regulations exist to ensure that only safe and effective products, produced in a defined and traceable way, reach consumers. However, ensuring compliance with these guidelines can be time-consuming and stressful. Pharmaceutical companies must navigate a complex regulatory system, including 21 CFR Part 11 requirements, data integrity guidelines (ALCOA++), and USP <1058>, which outlines the entire process of analytical instrument qualification.
Regulatory compliance is essential in the pharmaceutical industry. Regulations exist to ensure that only safe and effective products, produced in a defined and traceable way, reach consumers. However, ensuring compliance with these guidelines can be time-consuming and stressful. Pharmaceutical companies must navigate a complex regulatory system, including 21 CFR Part 11 requirements, data integrity guidelines (ALCOA++), and USP <1058>, which outlines the entire process of analytical instrument qualification.
Good Practice (GxP) regulations, including Good Manufacturing Practice (GMP/ICH Q7) requirements, form the framework around this. An instrument must be qualified against the customer’s User Requirement Specification (URS) using Installation Qualification and Operation Qualification (IQ/OQ) procedures and documentation. The customer must then perform regular testing as part of an ongoing Performance Qualification (PQ) schedule.
What are the challenges of compliance?
Performing measurements in a validated/regulated environment needs more care than ever before. In addition to 21 CFR Part 11 compliance, there is a requirement to consider the data integrity of measurements (ALCOA++), including long-term storage/accessibility of the data, and qualification of the analytical instrument itself. In some cases, the time taken to examine and approve measurements can exceed the sample processing time. This is part of the necessary framework that must be in place for the development and manufacture of safe pharmaceuticals. Our software, including our OmniTrust package, enables (with appropriate local IT infrastructure) 21 CFR Part 11 compliance and ALCOA++ data integrity guidance. This compliance is then tested through regular surveillance audits from the relevant regulatory bodies.
How can Malvern Panalytical help?
With many years’ experience of qualifying instruments and developing software used in regulatory environments, we have extensive knowledge in this area. As well as supplying necessities such as comprehensive IQ/OQ for our products, we:
- Supply standards for Performance Qualification (PQ)
- Supply test scripts to validate the 21 CFR Part 11 and data integrity features on your system
- Supply feature-rich software that can be used as part of a 21 CFR Part 11 and/or data integrity compliant solution
- Can consult on your custom validation needs, advise on GMP/ICH Q7 pharmaceutical qualification, and support you with any additional queries or requirements
USP <1058>: Analytical Instrument Qualification
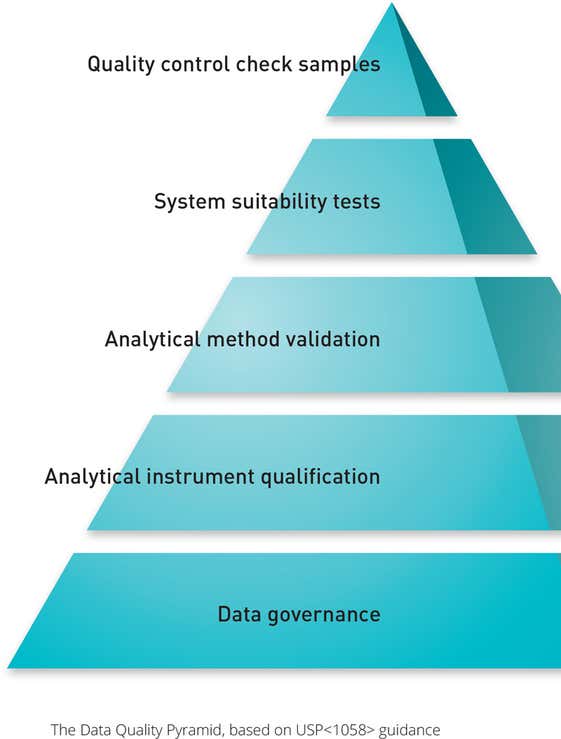
The requirements detailed in USP <1058> have highlighted the importance of IQ/OQ in the validation process. These procedures are now directly linked to the original User Requirement Specification (URS) for an instrument, along with its Performance Qualification (PQ) and Performance Verification (PV).
Operation Qualification must also now test that the instrument is fit for the end user's application, as well as working as the manufacturer designed it.
Malvern Panalytical offers a comprehensive IQ/OQ package for this purpose, and we are also happy to consult on additional testing needs.
Software for the regulated environment
Auditing a measurement can take time, so our software contains many features to make this process more efficient for our customers. It has been designed to be simple but powerful, saving time on implementation and in daily use, streamlining the workflow, and allowing complete confidence that no surprises will be discovered during an audit. This forms part of a 21 CFR Part 11 compliant solution (in conjunction with a robust IT policy).
Key software features include:
- Defining set roles for users
- Approval processes for signing measurements
- Recording reasons for aborting measurements
- Capturing audit trail entries for failed actions due to the use of incorrect credentials
- Audit of the security features for remote viewing
 Download the OmniTrust brochure: Malvern Panalytical's compliance solution for the regulated environment
Download the OmniTrust brochure: Malvern Panalytical's compliance solution for the regulated environment
Compliance Services
Standards
We supply a variety of reference and calibration standards for all your validation needs.
IQ/OQ and additional test scripts
Our Customer Support team can perform IQ/OQ services and supply validation test scripts to validate the 21 CFR Part 11 and data integrity features of the software.
Validation Services
We always welcome conversations about additional validation requirements with our customers.
We provide software, including our OmniTrust package, for use in the regulated environment, which benefits from enabling features such as audit trails, electronic signatures, and layered access.
Featured content

Demo at your desk – OmniTrust on Mastersizer 3000; Data integrity solution for the regulated environment
Featured products