As the pharmaceutical industry implements PAT and QbD, their symbiotic nature becomes increasingly obvious. Both are catalysts towards the longer term goals of continuous operation and real-time release - the realization of a transformed way of working. This article reviews changing practice within the pharmaceutical industry using the example of real-time particle size analysis to explore the analytical solutions needed and the benefits they deliver.
|
|
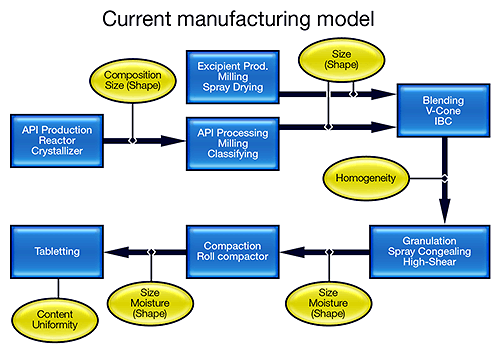
Despite its sophistication in many areas, the pharmaceutical industry has remained conservative in its production practices, largely because of the regulatory framework in which it operates. Batch processing is still the norm in manufacturing suites, where there is unlikely to be a centralized control room. Processes are broken down into a series of steps, with rigorous checks being applied after each one, to determine a material's suitability for the next stage. And there is little embedded automation.
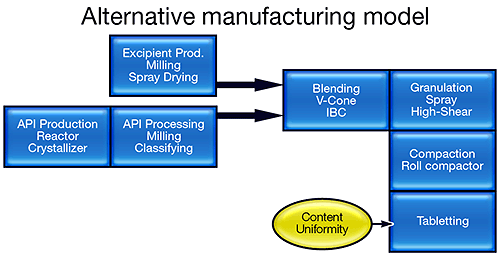
The FDA's vision is to transform this manufacturing model into one that more closely resembles that of the bulk chemical industry (see figure 1). Knowledge-based semi-continuous or continuous processing offers reduced risk, improved product quality and less waste. Such a transformation is, however, extremely challenging, and requires the industry to focus on processing in a completely different way. Research activity is escalating, with funding for initiatives, such as the Novartis-MIT Center for Continuous Manufacturing and a Rutgers-led university research consortium, underpinning the development of new manufacturing processes [1,2].
Continuous real-time analysis will be an essential element of new manufacturing processes and, as the PAT initiative recognizes, this will mean adopting technologies that are new to the pharmaceutical sector. Particle size, composition and homogeneity are just some of the variables for which reliable analytical solutions are needed. Real-time measurement allows processors to monitor and control product quality continuously rather than simply test for it at the end of the process. It therefore has the potential to drastically reduce the amount of waste produced and greatly improve equipment utilization. Developing manufacturing processes that enable the pharmaceutical industry to produce what it wants, when it wants, will have a major impact on process economics. In addition, continuous analysis has value beyond the production environment. As the full implications of Quality by Design are appreciated, it is becoming obvious that PAT will be equally important at the development stage.
Automated real-time analysis can significantly reduce the burden associated with establishing the design space - the operating window for regulatory approval. This is a core QbD activity. The suggested approach for pharmaceutical development now involves identifying critical product quality attributes, those that directly impact product performance, and then developing an understanding of the manufacturing variables that affect them.
Because PAT enables the investigation of these critical control parameters (CCPs) it accelerates the product development cycle. For example, with on-line or in-line particle size analysis it is possible to investigate the full impact of different milling variables in hours, rather than days or even weeks. Currently PAT instruments are making their way into R&D and pilot facilities as well as into full scale production. Trialling at process development scale serves a dual purpose, allowing the reliability, relevance and usefulness of the instrument to be assessed, while at the same time gathering data for further process optimization.
For continuous process monitoring the pharmaceutical industry needs solutions that meet a demanding set of constraints. The three primary requirements of a process analytical system are:
Firstly of course the analyzer must measure a defining parameter for the process. Returning to the example of milling, the goal is usually to produce material with a specific particle size and size distribution to ensure optimal processing downstream. Real-time particle size data are therefore highly relevant. This is also the case for a variety of other unit operations including granulation, roller compaction, spray drying and emulsification. In all these the particle size of the exiting material is critical, so continuous measurement is of great value.
Delivering measurement results in a process-relevant timeframe is where many analytical technologies fail. Two parameters are important when considering this issue - data acquisition time (DAT) and measurement acquisition time (MAT). DAT is the time taken for the instrument to collect a piece of data. MAT, on the other hand, is the time taken for the instrument to collect and analyze sufficient data to produce a measurement result. MAT is the more important of these two parameters since it will determine the effectiveness of monitoring and control. Some analytical techniques have a relatively short DAT but data analysis is arduous, so it takes some time to produce a usable measurement. This immediately compromises the applicability of the technology for process control. Consider the example of automated microscopy, where the time required for taking a picture is measured in milliseconds (DAT), but the need to analyze hundreds of thousands of particles for the result to have a statistical meaning extends the MAT of this technique to many minutes.
Pharmaceutical mills for example, have residence times typically in the order of seconds. The process time constants are of a similar magnitude, so the MAT of an instrument must be significantly less than this to capture the dynamics of the process. If the particle size of material produced in the mill can change significantly in a period of, say, a minute, then monitoring the process with an instrument that makes a measurement every ten minutes is far from ideal. Only an instrument with a MAT of less than a minute will be able to provide usable data for process control.
Finally, there is the issue of what constitutes a process appropriate device. The demands that continuous operation place on any analyzer are considerably more arduous than those which apply in the laboratory environment. Automation and reliability are critical, and ideally the device will incorporate self diagnostics. An additional requirement, unique to the pharmaceutical industry, is the need for cGMP/GAMP compliance. Communications between the device and controller are also important since linking the device with other analyzers into a centralized control system will increase its value (see page 6 - Using real-time analysis for automated control).
Laser diffraction is a proven technology for particle size measurement and is widely used across a range of different industries. It is fast, non-destructive and, since it is based on a 'first principles' mathematical analysis, requires no calibration. The technique lends itself to automation and has been successfully incorporated into robust instruments that meet the stringent requirements of the pharmaceutical processing environment. Its versatility means that the same measurement technology can be used for both wet and dry systems in the laboratory, on the pilot plant, and for full scale manufacture, simplifying scale-up and validation.
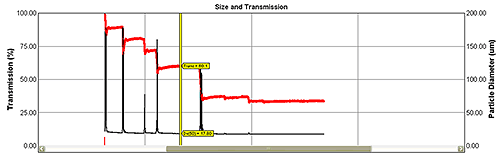
As laser light passes through a sample it is scattered by particles in a way that is dependent on their size. Particle size distribution can therefore be calculated from the detected diffraction pattern. The ability of an on-line particle size analyzer to function as a true real-time measurement device depends on it being able to produce accurate results in varying process conditions. If not accounted for, multiple scattering can compromise data accuracy for more concentrated particle streams. Patented algorithms incorporated into systems from Malvern Instruments address this issue, allowing accurate measurement in changing concentration conditions (see figure 2) and so extending the applicability of the technique.
|
Red trend line shows changes in light transmission from 90% to 30% with virtually no effect on size results (Dv(50) - black line).
Laser diffraction is not only an extremely fast measurement technique. The best on- and in-line systems make four measurements per second (MAT = 4 Hz) so even rapidly changing processes are monitored successfully, as illustrated in the following examples. It is also capable of processing relatively large volumes of sample - several kg/hr, thus making analysis of entire batches possible.
Spray drying is one unit operation for which particle size is a critical parameter. Figure 3 shows an in-line laser diffraction analyzer measuring the output from a small lab-scale dryer.

|
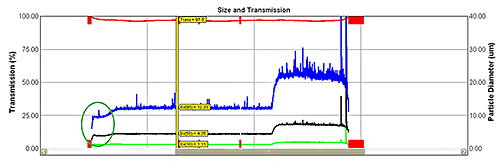
The instrument measures the entire product stream, producing data with high statistical significance. Its installation as close as possible to the exit minimizes time delays. A feedback control loop allows manipulation of either atomizer pressure or feed to maintain particle size at a given set point. Increasing feed rate produces coarser particles; increasing atomization pressure has the opposite effect. Figure 4 shows data from the in-line system.
|
Data within the green oval show an initial stabilization phase after which particle size becomes steady. The change in set point to a coarser material is clear. The process returns to steady operation soon after the change has been made, demonstrating effective control.
|
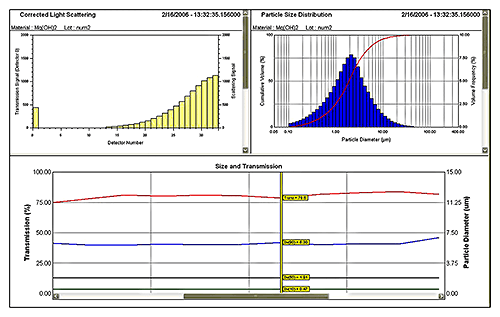
Figure 5 shows data from a laser diffraction particle size analyzer measuring a wet process. Here an on-line system is monitoring a Mg(OH)2 suspension produced by wet milling. A 2 mL sample is extracted from the process line, automatically diluted and prepared (see figure 6) before analysis. Sample extraction, dilution and measurement are controlled by SCADA interface. The system produces a measurement every few minutes giving a MAT low enough to provide effective monitoring and control.

|
Clearly real-time analysis has a vital role in the transformation of the pharmaceutical industry. At the development stage it provides the data required to cost-effectively implement a QbD approach, while at the manufacturing level it will underpin the shift towards continuous processing and real-time release.
The PAT initiative promotes the uptake of new analytical technologies but as yet has not had a dramatic impact on full-scale manufacturing. This will come as systems prove their worth at the pilot stage, and the integration of different analyzers within a centralized control system becomes a part of implementing industrial guidelines like ICH Q10 Pharmaceutical Quality Systems. The standardization efforts promoted by FDA, EMEA and groups like ISPE and ICH result in industrial collaborations like OPC ADI working group (see below) that bring different equipment vendors together.
For some variables, continuous analysis in a relevant time frame remains the challenge; for others appropriate systems are already well-established and commercially available. Laser diffraction for particle size analysis, for example, is an extremely suitable technique, for both wet and dry systems. Fast measurement provides effective monitoring for even the most rapidly changing processes. Exploiting the true potential of technologies such as these, through automated control, will enable the pharmaceutical industry to experiment and manufacture in a timely and cost-effective way.
The commitment to transforming manufacturing practice within the pharmaceutical industry presents an enormous opportunity. Building on the experience of other industries, it should be possible to implement the very best processing methods using the latest technologies. The change also provides a major incentive to invest in developing new solutions where these are required.
Optimum process control demands real-time analysis and effective data transfer. Isolated analyzers provide continuous monitoring of an individual variable but linking analyzers in an automated centralized control system adds considerable power. A challenge here is the current absence of any standard for interface software. Control software designed for use with a range of analyzers is commercially available, but it relies on using the proprietary driver of the individual device. Implementation costs are high because each solution is tailor made.
Now, the OPC Analyzer Device Integration working group aims to address this issue. The group was established by ABB and currently has 21 members, including representatives from leading pharmaceutical producers, instrument vendors and automation companies. Their aim is to develop a model that will allow equipment vendors to produce a standard software interface that will expose the capabilities of both process and laboratory instruments.
The model will utilize OPC Unified Architecture (UA) since it is an effective, universally accepted, platform-neutral option. Establishing the standard will reduce the implementation costs associated with PAT, and will help users to more easily exploit the full potential of real-time analysis. The working group hopes to develop a solution through a multidisciplinary approach, using the perspectives and skills of everyone affected by the issue.