
MicroCal ITC range
The "Gold Standard" for measuring binding affinity and stoichiometry
Label-free measurement of the binding affinity and thermodynamics of biomolecular interactions to understand function and mechanisms at a molecular level
Isothermal Titration Calorimetry (ITC) is a label-free quantification technique used in studies of a wide variety of biomolecular interactions. It works by directly measuring the heat that is either released or absorbed during a biomolecular binding event.

The "Gold Standard" for measuring binding affinity and stoichiometry
ITC is the only technique that can simultaneously determine all binding parameters in a single experiment. Requiring no modification of binding partners, either with fluorescent tags or through immobilization, ITC measures the affinity of binding partners in their native states.
Measuring heat transfer during binding enables accurate determination of binding constants (KD), reaction stoichiometry (n), enthalpy (∆H) and entropy (ΔS). This provides a complete thermodynamic profile of the molecular interaction. ITC goes beyond binding affinities and can elucidate the mechanisms underlying molecular interactions. This deeper understanding of structure-function relationships enables more confident decision-making in hit selection and lead optimization.
Isothermal Titration Calorimetry is used to measure reactions between biomolecules. The methodology allows determination of the binding affinity, stoichiometry, and entropy and enthalpy of the binding reaction in solution, without the need to use labels.
When binding occurs, heat is either absorbed or released and this is measured by the sensitive calorimeter during gradual titration of the ligand into the sample cell containing the biomolecule of interest.

In the microcalorimeter there are two cells, one of which contains water and acts as a reference cell, the other contains the sample. The microcalorimeter needs to keep these two cells at exactly the same temperature. The heat sensing devices detect temperature difference between the cells when binding occurs and give feedback to the heaters, which compensate for this difference and return the cells to equal temperature.
The reference cell and the sample cell are set to the desired experimental temperature. The ligand is loaded into a syringe which sits in a very accurate injection device. The injection device is inserted into the sample cell containing the protein of interest. A series of small aliquots of ligand are injected into the protein solution. If there is a binding of the ligand to the protein, heat changes of a few millionths of a degree Celsius are detected and measured.
As the first injection is made, the microcalorimeter measures all heat released until the binding reaction has reached equilibrium. The quantity of heat measured is in direct proportion to the amount of binding.
In the example below, the reaction is exothermic, which means the sample cell becomes warmer than the reference cell and causes a downward peak in the signal. As the temperature of the two cells comes back to being equal, the signal returns to its starting position. The second small aliquot of the ligand is injected into the sample cell and once again the microcalorimeter compensates for the small heat changed detected.
The molar ratio between the ligand and protein is gradually increased through a series of ligand injections. The protein gets more and more saturated, less binding of the ligand occurs and the heat change starts to decrease until ultimately the sample cell contains an excess of ligand versus protein, bringing the reaction towards saturation.
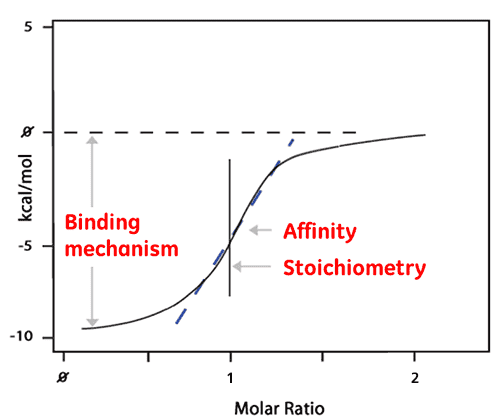
The area of each peak is then integrated and plotted versus the molar ratio of ligand to protein. The resulting isotherm can be fitted to a binding model from which the affinity (KD) is derived. The molar ratio at the center of the binding isotherm give us the reaction stoichiometry. The plot shown below is an example of a 1:1 binding reaction.
The enthalpy (ΔH) is also derived directly from the isotherm and is the amount of heat released per Mole of ligand bound. This means that a single ITC experiment delivers a wealth of information about the binding reaction which helps understand the nature of the interaction and explore the thermodynamic drivers.
ITC is widely used in drug discovery and development for:

PEAQ-ITCThe Gold Standard for measuring affinity and stoichiometry |

MicroCal PEAQ-ITC AutomatedFully automated, hands free ITC for increased productivity |
|
|---|---|---|
| Measurement type | ||
| Label-free analysis | ||
| Binding affinity | ||
| Technology | ||
| Isothermal Titration Calorimetry (ITC) | ||
| Sample throughput | Up to - 12per 8h day | Up to - 42per 24h day |