In this application note, the Malvern MicroCal VP-Capillary DSC has been used for rapid screening of buffers in preformulation development and for the optimization of storage conditions of antibodies during process development. The results correspond well with those obtained by other more time-consuming methods.
The use of biotherapeutics, such as antibodies and other protein molecules, to treat diseases is a rapidly growing field in the pharmaceutical industry. Biotherapeutics are often required at high concentrations and multiple doses, hence a manufacturer must produce kilogram quantities or more of the protein drug. The manufacturing process for biotherapeutics involves protein expression in thousands of liters of bioreactor medium followed by a purification process using large-scale chromatography columns and filtration systems.
The stability of the protein to process conditions, reversibility of conformational changes, and any propensity to form aggregates depends on factors such as pH and buffer composition. A thorough understanding of these factors is important for the selection of process conditions, formulation and development of analysis methods. Steps in the antibody purification process that could render a protein unstable include low pH elution step from protein A column, low pH hold step for viral inactivation and any stage involving pH and/or ionic strength adjustment, including final formulation.
Differential Scanning Calorimetry (DSC) provides information on the thermal stability of a protein under conditions of different pH and cosolutes, by monitoring thermal transition midpoints, Tm. A higher Tm reflects higher thermal stability, which correlates well with long term stability. This application note describes how Diosynth Biotechnology uses thermal stability data obtained from DSC to characterize the stability of an antibody during initial pH and buffer screening for preformulation development and for optimization of the low pH viral inactivation used in the manufacturing process. Low pH viral inactivation is desirable for protein manufacturing provided it does not cause any decrease in protein stability.
DSC was performed using a Malvern MicroCal VP-Capillary DSC system. In the preformulation development study a range of buffers with pH between 3 and 8 were used. The protein (antibody X) was stored in each buffer, and assays were performed immediately (t = 0) and after one week of storage (t = 1 week).
For the optimization of purification conditions, DSC thermograms of antibody Y (in a neutral Tris buffer containing NaCl and EDTA, pH 7.4) were studied and compared to a pH 3 citrate buffer and also the citrate buffer adjusted to pH 6 with 2 M Tris, pH 9.0.
Thermograms of the buffer alone were subtracted from each protein prior to analysis using Origin™ 7.0 software equipped with MicroCal VP-Capillary DSC analysis software.
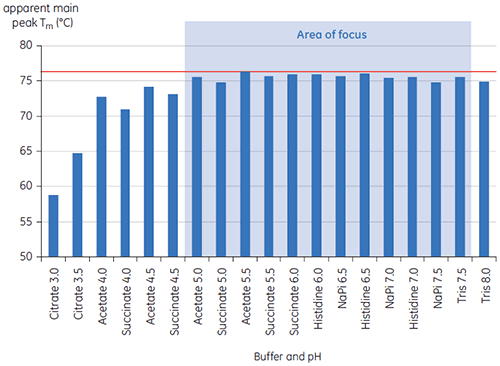
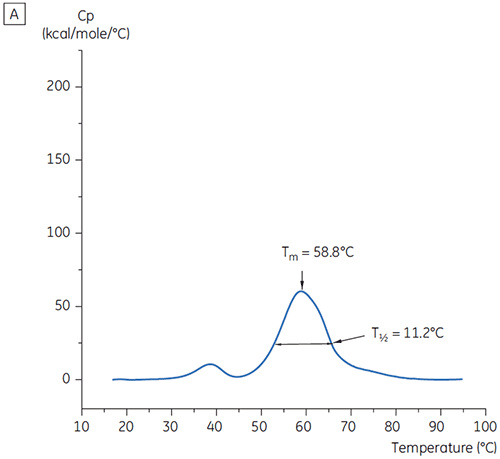
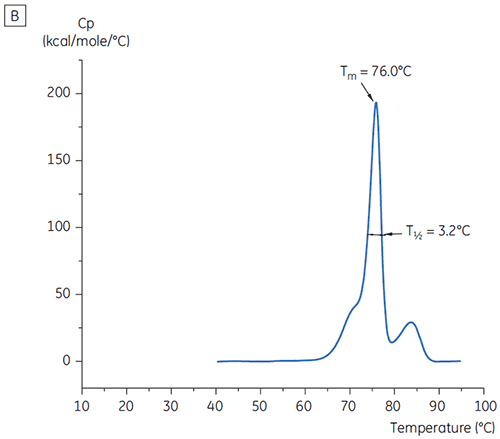
The values of the main Tm peak at t = 0 for antibody X in the initial study of 19 buffers for preformulation development are shown in Figure 1. The DSC thermograms for the antibody in two of these buffers are shown in Figure 2. From the Tm values, the most stable buffer conditions are found between pH 5.0 and pH 7.5. At t = 0, other analytical methods (UV, size exclusion chromatography (SEC), light scattering, and SDS-PAGE) showed much less discrimination between the buffer conditions as compared to DSC (data not shown).
|
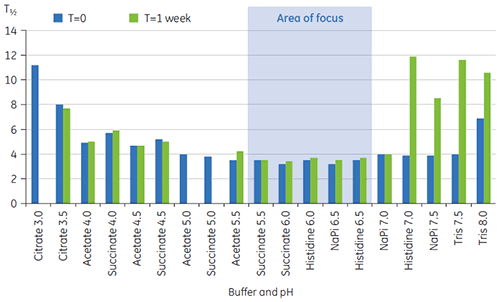
T½ values were used to further discriminate between conditions, as indicated in Figure 2. T½ is the peak width at half maximal height for the major transition in the DSC thermogram and typically reflects the co-operativity of the thermal transition. A lower T½ value can indicate a more compact structure and is therefore preferred for formulations. Here, the lowest T½ values were found for buffers with pH values between 5.5 and 6.5 (Figure 3).
|
|
|
Citrate buffer at pH 3 is a candidate for use for both the elution of the antibody from the protein A affinity column and for the subsequent low-pH hold step for viral inactivation. Since the majority of proteins tend to become unstable under prolonged exposure to such a low pH, the pH must be raised immediately after the viral inactivation step.
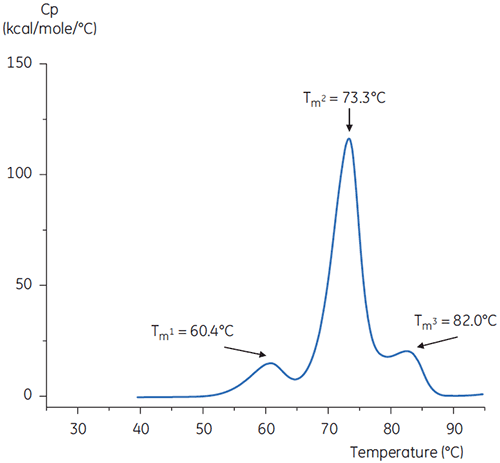
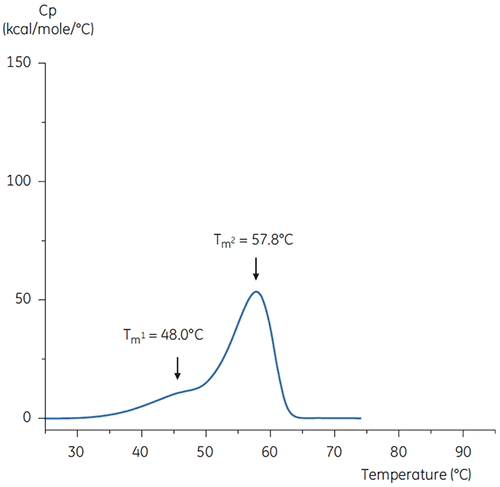
The DSC scans of the antibody in buffer at neutral pH and at pH 3 are shown in Figures 4 and 5.
|
|
The differences in shape and pattern of the two thermograms demonstrate loss of thermal stability of the antibody in the pH 3.0 buffer. The peak height of the Tm2 transition is lower, the peak is broader and the definition between the first and second transitions in pH 3.0 is less pronounced as compared to pH 7.4.
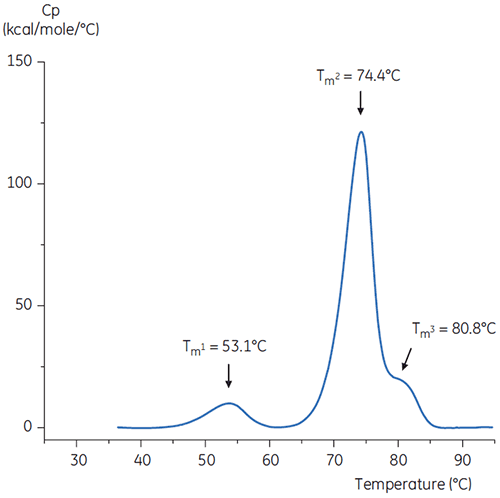
To imitate the pH neutralization step, the pH of the antibody solution was adjusted to pH 6.0 with a concentrated Tris solution at pH 9. The thermogram of the resulting antibody solution is shown in Figure 6. Here, an increased thermal stability relative to the antibody in pH 3.0 buffer is seen. The Tm2 and Tm3 of the antibody in the pH 6.0 solution are similar to Tm2 and Tm3 of the antibody in the reference buffer. The overall shape, peak definition, and peak width for the pH 6 antibodies are also similar to the reference situation at pH 7.4.
Neutralization can also be done in the presence of stabilizing excipients, such as histidine. In this case, the thermogram of the antibody neutralized in the presence of histidine was almost identical to Figure 6, indicating that for this particular antibody, histidine did not provide any significant stabilizing effect.
|
This study shows that DSC could be used to quickly optimize pH and buffer conditions for preformulation development. These data were used to rank appropriate buffers and pH interval for the subsequent excipient screening, reducing the number of exploratory conditions considerably.
DSC can also be used for checking the stability of an antibody during low pH inactivation hold step and following adjustment of pH from 3.0 to 6.0. This kind of stability information is useful in designing and optimizing processes for biopharmaceutical manufacturing.
This data was kindly provided by Dr Kathrine E. Bowers, FUJIFILM Diosynth Biotechnologies USA Inc.