This document illustrates how the technique can be used to examine the stability of individual domains in multi-domain proteins and guide process development for antibodies and other proteins.
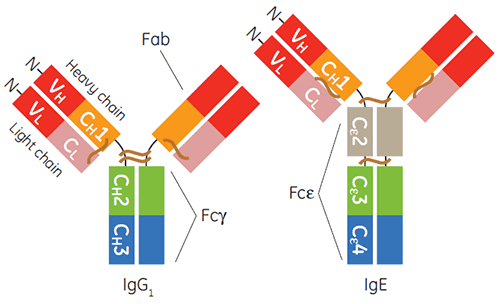
The production and purification of immunoglobulins of the gamma isotype, particularly human IgG1, for diagnostic or therapeutic applications is now fairly routine. In the past decade IgE-based therapeutics have also gained momentum (1-3). IgE is important for host defense against parasites and for protective inflammation. Yet, IgE-mediated signaling through its receptors is also a focal point of inflammatory allergic disease (4). The constant region of IgE, is a homodimer containing duplicate pairs of three unique Ig-fold domains (Cε2, Cε3, and Cε4), and is responsible for binding its two receptors, FcεRI and CD23, also known as FcεRII (Figure 1).
This application note focuses on the utility of differential scanning calorimetry (DSC) to inform multiple aspects of the biotherapeutic development processes of IgG and IgE. DSC enables the study of protein unfolding rapidly and with out the use of labelling or artificial probes. The technique determines the heat absorbed by the sample as the protein unfolds, giving a measure of its thermostability and an indication of long-term stability.
In particular in the work described here, it is shown that DSC provides insights for handling, purifying, and formulating IgG and IgE drug products. The ability of circular dichroism (CD) to contribute to these findings is contrasted to what can be discerned using DSC. DSC enables the investigation of protein stability at the level of individual domains within multi-domain proteins, an aspect that is less transparent in data obtained by CD.
|
The experiments were performed using the Malvern MicroCal VP-Capillary DSC.
Purified Fcε, Fcγ and Fcγ-Cε2 proteins were generated as described previously (5). The entire set of capillary DSC experiments, more than 400 scans at different pH values, were performed over a four-month period with very little effort. It took approximately three hours labor to set up the experiments for Fcε and Fcγ, including the measurement of protein concentrations, dilutions, and setup of the plate. The remainder of the experiment was performed by the automation of MicroCal VP-Capillary DSC. Full details described in (5).
Broad pH/salt stability is a prerequisite for many affinity protein purification processes performed in the industrial setting. Poor tolerance to unusual pH or salt conditions can result in aggregated or non-functional protein. The tolerance of Fcε to various pH/salt conditions is important information for determining an appropriate and scalable purification scheme for IgE/Fcε-containing proteins. To study the effect of pH on the secondary structure of Fcε, CD spectra were taken of the protein under buffer conditions ranging from pH 4.5 to 7.4.
Between pH 5.2 and 7.4, the spectra of Fcε were identical and contained a single minimum between 216 and 217 nm, indicative of significant ß-sheet and typical of Ig-domains. At pH 5, the Fcε spectrum shifted in a random coil direction (the minimum shifted towards 200 nm), and at pH 4.5, the spectrum suggests the protein is predominantly random coil (5). Based on the pH-dependent unfolding, we investigated whether Fcε may have an attenuated stability between pH 7.0 and 4.5.
Thermal denaturation of Fcε at various pH values was monitored by far-UV CD. At pH 7.0, there was one transition for the unfolding of all three domains (Cε2-4). A similar transition was observed at pH 6.0, though the apparent Tm decreased by 1°C. Thermal unfolding of Fcε at pH 5.2 resulted in a much broader transition that began 6°C lower than at neutral pH. Only at pH 4.8 were two transitions clearly evident (5).
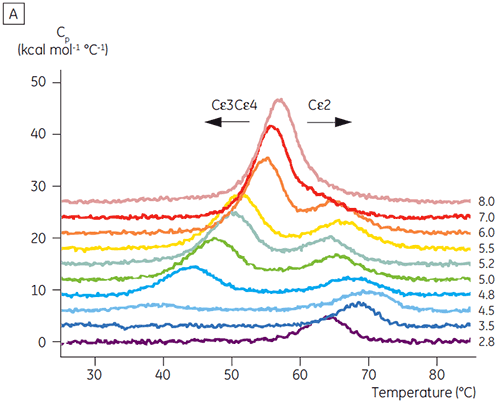
Based on the initial CD results, detailed pH-dependent stability studies were initiated for both Fcε and Fc γ using DSC. The unfolding transitions of both Fcε and Fcγ were found to be irreversible and scan rate dependent (data not shown), suggesting that irreversible aggregation affects the apparent Tm values of both proteins (6,7). Unlike what could be determined using CD, Fcε was shown to contain two independent unfolding transitions at all pH values below 8.0 (Figure 2A). One of these transitions was destabilized at low pH and high NaCl while the other was not.
|
|
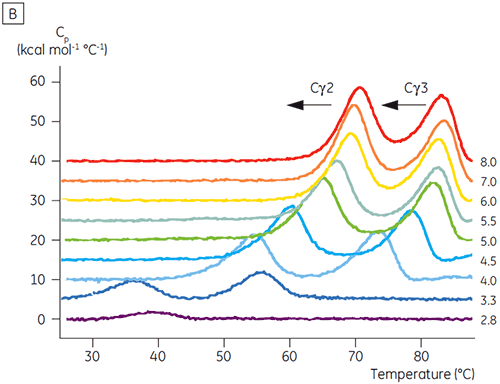
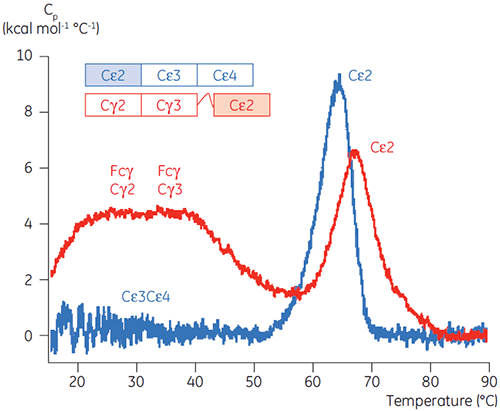
The domains involved in the pH-sensitive transition were completely unfolded at pH 4.5 as expected based on the structural data obtained using CD, demonstrating how DSC can be important not only for understanding the stability of folded domains, but their folding status as well. Fcγ (from IgG1) was shown to unfold via two separate transitions; the low temperature transition belonging to the Cγ2 domain and the high temperature transition belonging to the Cγ3 domain (Figure 2B). The Cγ2 transition was identified by the effect that deglycosylation had on its thermostability (unpublished results) and the Cγ3 transition by its unusually high thermostability (8). Both Fcγ domains were shown to be pH and NaCl sensitive. Unlike Fcε, the Fcγ domains did not become intrinsically unfolded until the pH was reduced below 3.0, suggesting why antibodies elute from protein A media at pH values below 3.5, and why cation exchange chromatography may be a suitable purification technique for IgG (Figure 3).
|
|
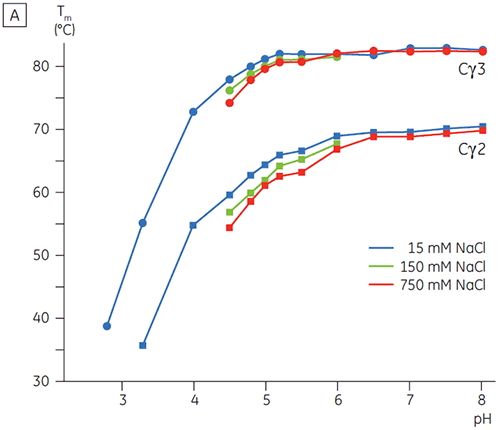
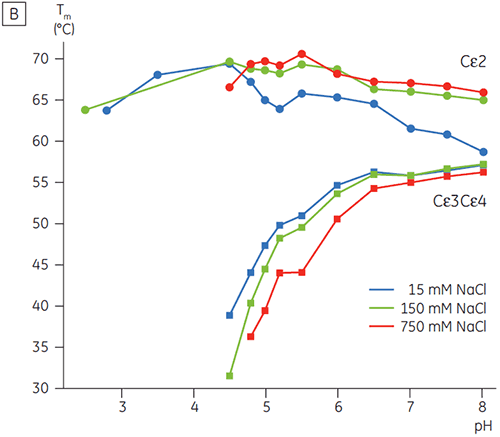
In the presence of high salt, the Cγ2 and Cγ3 domains of Fcγ and the Cε3 and Cε4 domains of Fcε were destabilized. This was seen as a small shift in their Tm values at 150 and 750 mM NaCl relative to 15 mM NaCl in the intermediate pH range between 5.0 and 7.0 (Figure 3A). These small stability differences are unlikely to have a major affect on the in vitro half-life of Fcγ within this pH range because the Tm of both the Cγ2 and Cγ3 domains remained above 60°C.
The pH-sensitive domains of Fcε were identified as the receptor binding domains (Cε3 and Cε4) by performing DSC experiments with an Fcγ-Cε2 fusion protein. One domain of both Fcγ-Ce2 and Fcε remains stably folded at pH 2.5 (Figure 4). Based on the experiments described above, it is known that the Fcγ domains are intrinsically unfolded below pH 3.0. By default, this identifies the Cε2 domain of Fcε as the pH insensitive domain. These results were confirmed by limited proteolysis of Fcε at pH 4.5 (5).
|
In high salt, the Cε2 domain of Fcε was slightly more thermostable. Cε2 was especially stabilized at neutral pH and 750 mM NaCl with a Tm more than 7°C higher than the Tm measured in 15 mM NaCl (Figure 3B). In contrast, NaCl significantly destabilized the Cε3Cε4 domains between pH 5 and 6 (Figure 3B). Cε3Cε4 began to unfold at pH 5.0 in low salt. In high salt, the unfolding transition was shifted 0.5 pH units to pH 5.5, precluding the use of cation exchange chromatography as a viable purification step for IgE or Fcε containing proteins.
In this study, we showed that Fcε demonstrated an unusual pH-sensitivity that resulted in the unfolding of its receptor binding domains at 2 pH units higher than what was observed for Fcγ (i.e., pH 5.0). The pH/salt sensitivity of Fcε determined by DSC provides valuable information for choosing purification strategies, handling procedures, and formulations for IgE-based proteins and suggests that standard IgG protocols will not be amenable. The pH stability data for Fcγ also suggests a likely mechanism for IgG time-dependent aggregation during standard affinity purifications (eg. most commonly on protein A media) that include low pH elution and maybe also hold steps.
This application note was kindly provided by Dr Stephen Demarest, Biogen Idec, San Diego CA.
1. Zhu, D. et al. A novel human immunoglobulin Fcγ Fcε bifunctional fusion protein inhibits Fcε RI-mediated degranulation. Nature Med. 8, 518–521 (2002).
2. Zhu, D. et al. A chimeric human-cat fusion protein blocks cat-induced allergy. Nature Med. 11, 446–449 (2005).
3. Belostotsky, R. and Lorberboum-Galski, H. Utilizing Fcε-Bak chimeric protein for studying IgE-FcεRI interactions. Clin. Immunol. 110, 89–99 (2004).
4. Gould, H.J. et al. The biology of IgE and the basis of allergic disease. Annu. Rev.Immunol. 21, 79–628 (2003).
5. Demarest, S.J. et al. An intermediate pH unfolding transition abrogates the abilityof IgE to interact with its high affinity receptor FcεRIα. J. Biol. Chem. 281, 30755–30767 (2006).
6. Sánchez-Ruiz, J.M. et al. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry 27, 1648–1652 (1988).
7. Vermeer, A.W. and Norde, W. The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophys. J. 78, 394–404 (2000).
8. Demarest, S.J. et al. Optimization of the antibody C(H)3 domain by residue frequency analysis of IgG sequences. J. Mol. Biol. 335, 41–48 (2004).